Abstract
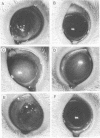
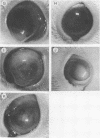
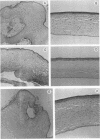
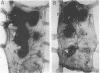
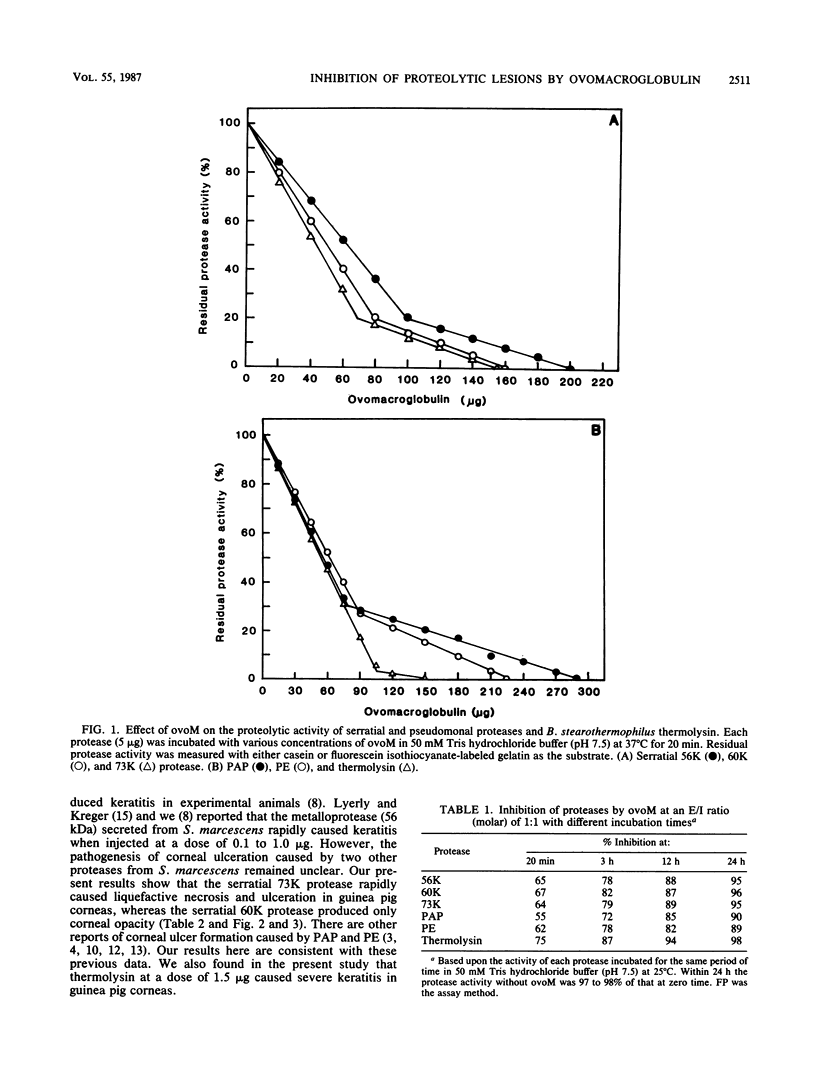
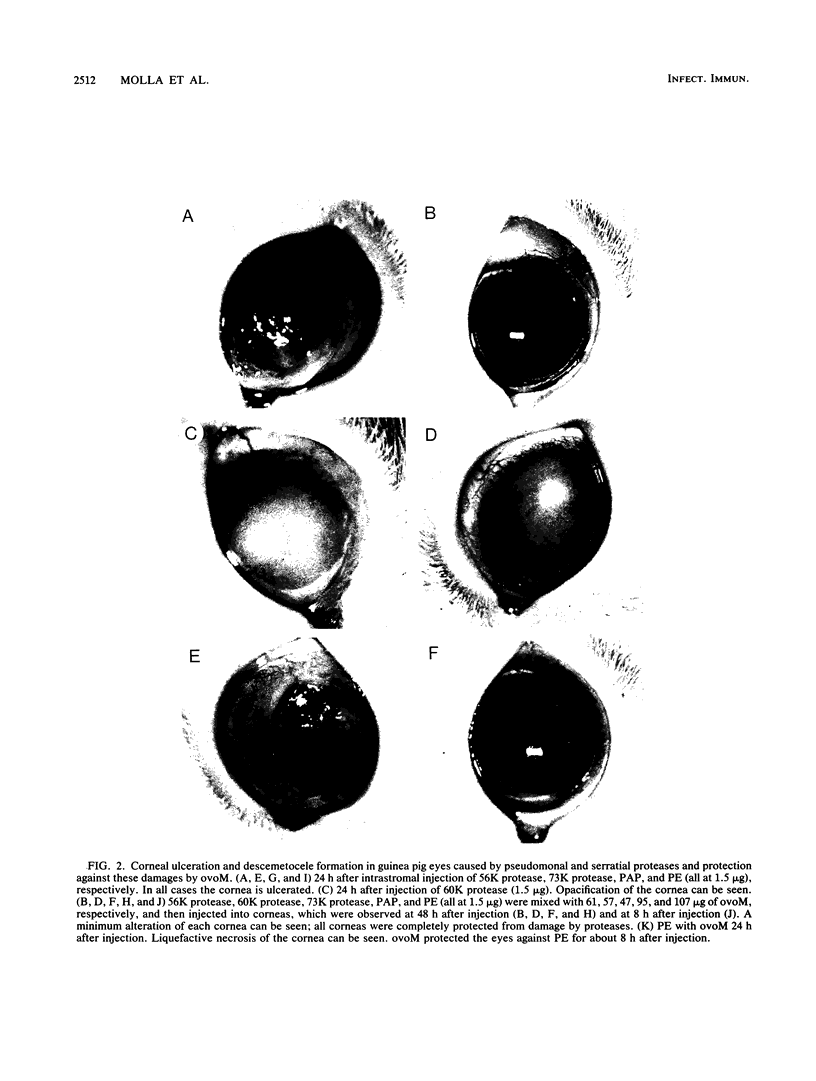
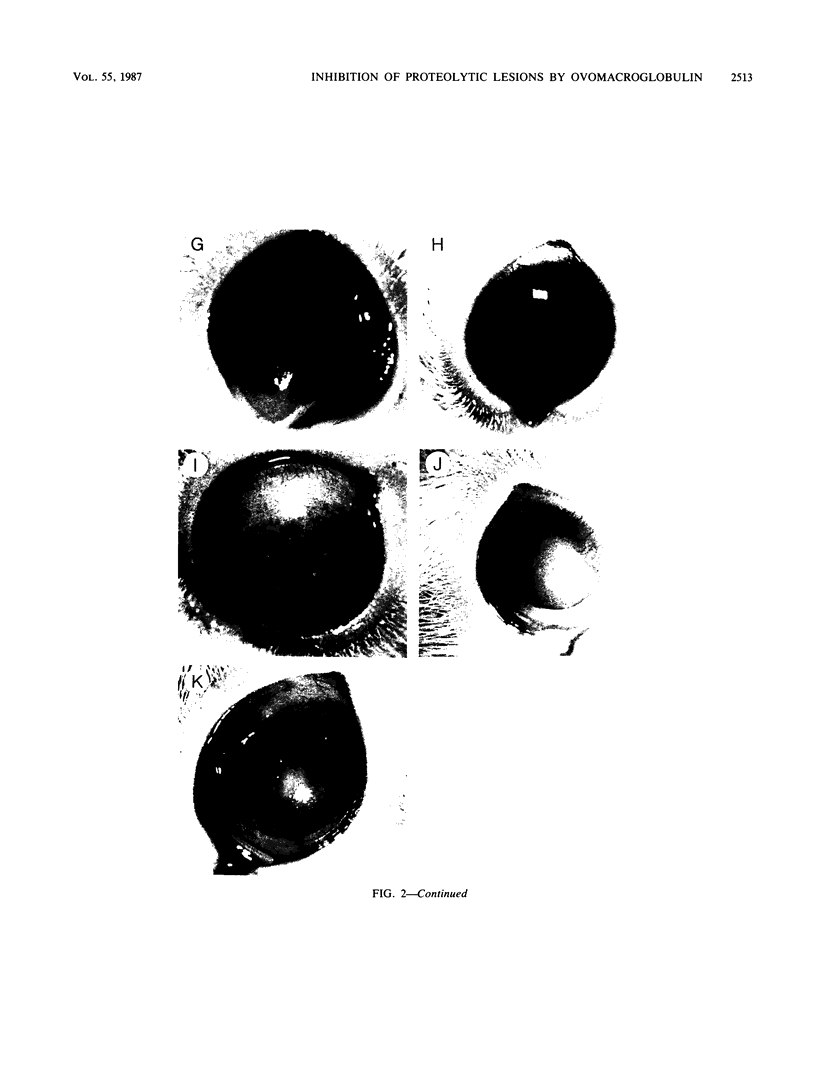
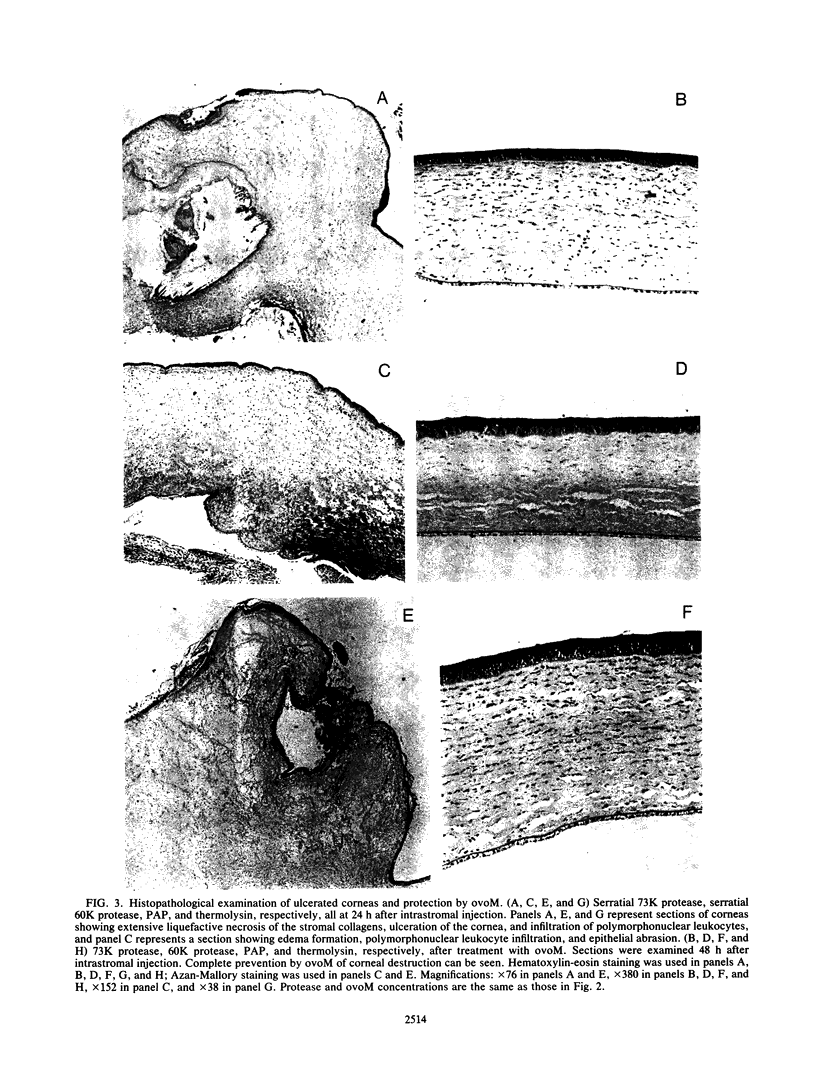
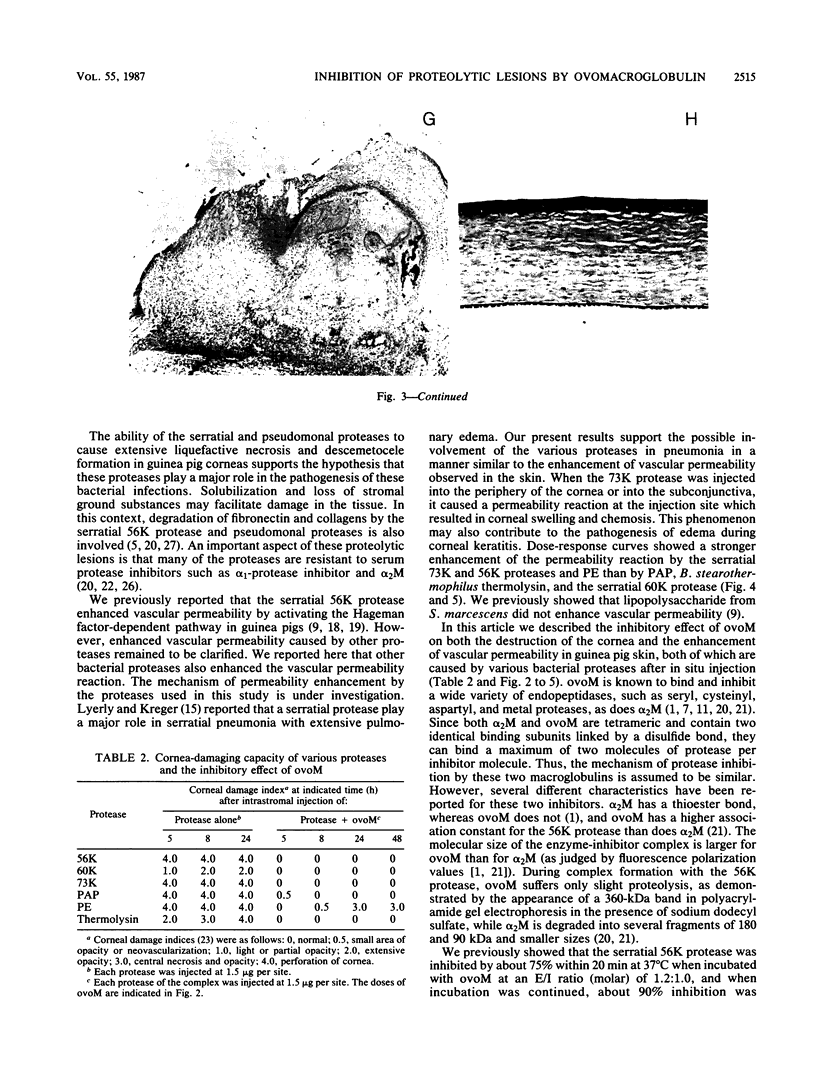
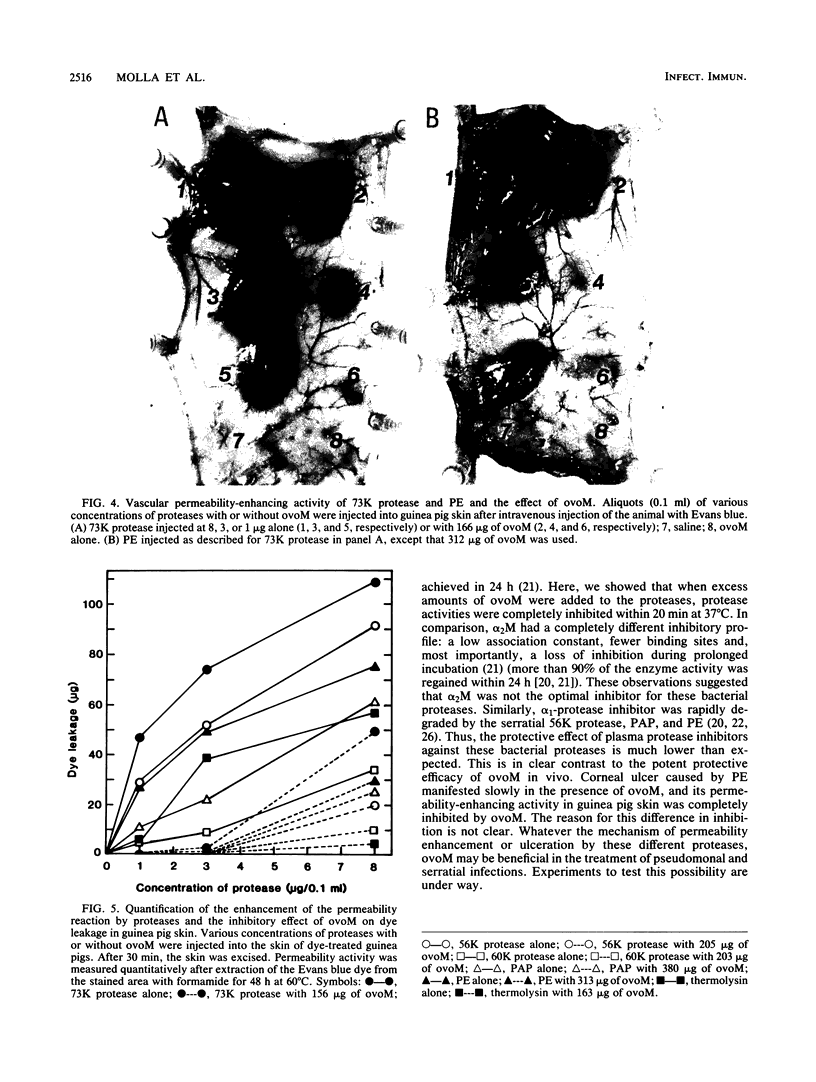
The pathogenicities of three proteases from Serratia marcescens, two proteases from Pseudomonas aeruginosa, and one thermolysin from Bacillus stearothermophilus were examined. All proteases tested caused acute liquefactive necrosis of the cornea and descemetocele formation in guinea pig eyes after intrastromal injection, with the exception of the 60-kilodalton protease from S. marcescens, which produced only an opaque lesion. When injected into guinea pig skin, the protease also enhanced vascular permeability, which was followed by edema formation. The permeability-enhancing activity of the proteases increased in parallel with the concentration of the enzymes. When tested in vitro for its effect on these bacterial proteases, chicken egg white ovomacroglobulin (ovoM) inhibited the enzymatic activity of all the proteases after a short incubation period at an enzyme/inhibitor ratio (molar) of 1:1 to 1:4 or at a lower concentration after a longer incubation period. Such treatment of the proteases with chicken egg white ovoM before injection intrastromally into the eyes or intradermally into the clipped flanks of guinea pigs protected the cornea from destruction or completely prevented the permeability reaction and edema formation. No inhibitory effects of plasma protease inhibitors against these bacterial proteases were noted. Since the proteases are critical in the pathogenic processes caused by the bacteria, these results suggest a beneficial effect of ovoM against bacterial infections.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa H., Osada T., Ikai A. Unusual properties of crocodilian ovomacroglobulin shown in its methylamine treatment and sulfhydryl titration. Arch Biochem Biophys. 1986 Feb 1;244(2):447–453. doi: 10.1016/0003-9861(86)90612-0. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- Brown S. I., Bloomfield S. E., Tam W. The cornea-destroying enzyme of Pseudomonas aeruginosa. Invest Ophthalmol. 1974 Mar;13(3):174–180. [PubMed] [Google Scholar]
- FISHER E., Jr, ALLEN J. H. Corneal ulcers produced by cell-free extracts of Pseudomonas aeruginosa. Am J Ophthalmol. 1958 Jul;46(1 Pt 2):21–27. doi: 10.1016/0002-9394(58)90030-8. [DOI] [PubMed] [Google Scholar]
- Heck L. W., Morihara K., McRae W. B., Miller E. J. Specific cleavage of human type III and IV collagens by Pseudomonas aeruginosa elastase. Infect Immun. 1986 Jan;51(1):115–118. doi: 10.1128/iai.51.1.115-118.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiby N. Pseudomonas aeruginosa infection in cystic fibrosis. Relationship between mucoid strains of Pseudomonas aeruginosa and the humoral immune response. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Aug;82(4):551–558. [PubMed] [Google Scholar]
- Ikai A., Kitamoto T., Nishigai M. Alpha-2-macroglobulin-like protease inhibitor from the egg white of cuban crocodile (Crocodylus rhombifer). J Biochem. 1983 Jan;93(1):121–127. doi: 10.1093/oxfordjournals.jbchem.a134145. [DOI] [PubMed] [Google Scholar]
- Kamata R., Matsumoto K., Okamura R., Yamamoto T., Maeda H. The serratial 56K protease as a major pathogenic factor in serratial keratitis. Clinical and experimental study. Ophthalmology. 1985 Oct;92(10):1452–1459. doi: 10.1016/s0161-6420(85)33855-1. [DOI] [PubMed] [Google Scholar]
- Kamata R., Yamamoto T., Matsumoto K., Maeda H. A serratial protease causes vascular permeability reaction by activation of the Hageman factor-dependent pathway in guinea pigs. Infect Immun. 1985 Jun;48(3):747–753. doi: 10.1128/iai.48.3.747-753.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaharajo K., Abe C., Homma J. Y., Kawano M., Goto E. Corneal ulcers caused by protease and elastase from Pseudomonas aeruginosa. Jpn J Exp Med. 1974 Oct;44(5):435–442. [PubMed] [Google Scholar]
- Kitamoto T., Nakashima M., Ikai A. Hen egg white ovomacroglobulin has a protease inhibitory activity. J Biochem. 1982 Nov;92(5):1679–1682. doi: 10.1093/oxfordjournals.jbchem.a134097. [DOI] [PubMed] [Google Scholar]
- Kreger A. S., Gray L. D. Purification of Pseudomonas aeruginosa proteases and microscopic characterization of pseudomonal protease-induced rabbit corneal damage. Infect Immun. 1978 Feb;19(2):630–648. doi: 10.1128/iai.19.2.630-648.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger A. S., Griffin O. K. Physicochemical fractionation of extracellular cornea-damaging proteases of Pseudomonas aeruginosa. Infect Immun. 1974 May;9(5):828–834. doi: 10.1128/iai.9.5.828-834.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lyerly D. M., Kreger A. S. Importance of serratia protease in the pathogenesis of experimental Serratia marcescens pneumonia. Infect Immun. 1983 Apr;40(1):113–119. doi: 10.1128/iai.40.1.113-119.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H. Assay of proteolytic enzymes by the fluorescence polarization technique. Anal Biochem. 1979 Jan 1;92(1):222–227. doi: 10.1016/0003-2697(79)90649-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Maeda H., Takata K., Kamata R., Okamura R. Purification and characterization of four proteases from a clinical isolate of Serratia marcescens kums 3958. J Bacteriol. 1984 Jan;157(1):225–232. doi: 10.1128/jb.157.1.225-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Yamamoto T., Kamata R., Maeda H. Enhancement of vascular permeability upon serratial infection: activation of Hageman factor--kallikrein--kinin cascade. Adv Exp Med Biol. 1986;198(Pt B):71–78. doi: 10.1007/978-1-4757-0154-8_9. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Yamamoto T., Kamata R., Maeda H. Pathogenesis of serratial infection: activation of the Hageman factor-prekallikrein cascade by serratial protease. J Biochem. 1984 Sep;96(3):739–749. doi: 10.1093/oxfordjournals.jbchem.a134892. [DOI] [PubMed] [Google Scholar]
- Molla A., Matsumoto K., Oyamada I., Katsuki T., Maeda H. Degradation of protease inhibitors, immunoglobulins, and other serum proteins by Serratia protease and its toxicity to fibroblast in culture. Infect Immun. 1986 Sep;53(3):522–529. doi: 10.1128/iai.53.3.522-529.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla A., Oda T., Maeda H. Different binding kinetics of Serratia 56K protease with plasma alpha 2-macroglobulin and chicken egg white ovomacroglobulin. J Biochem. 1987 Jan;101(1):199–205. doi: 10.1093/oxfordjournals.jbchem.a121892. [DOI] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H., Oda K. Protease and elastase of Pseudomonas aeruginosa: inactivation of human plasma alpha 1-proteinase inhibitor. Infect Immun. 1979 Apr;24(1):188–193. doi: 10.1128/iai.24.1.188-193.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman D. E., Burns R. P., Iglewski B. H. Corneal infections in mice with toxin A and elastase mutants of Pseudomonas aeruginosa. J Infect Dis. 1980 Oct;142(4):547–555. doi: 10.1093/infdis/142.4.547. [DOI] [PubMed] [Google Scholar]
- Udaka K., Takeuchi Y., Movat H. Z. Simple method for quantitation of enhanced vascular permeability. Proc Soc Exp Biol Med. 1970 Apr;133(4):1384–1387. doi: 10.3181/00379727-133-34695. [DOI] [PubMed] [Google Scholar]
- Virca G. D., Lyerly D., Kreger A., Travis J. Inactivation of human plasma alpha 1-proteinase inhibitor by a metalloproteinase from Serratia marcescens. Biochim Biophys Acta. 1982 Jun 4;704(2):267–271. doi: 10.1016/0167-4838(82)90155-8. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Bass J. A. Role of fibronectin in the prevention of adherence of Pseudomonas aeruginosa to buccal cells. J Infect Dis. 1981 Jun;143(6):784–790. doi: 10.1093/infdis/143.6.784. [DOI] [PubMed] [Google Scholar]