Abstract
New Caledonia has generally been considered a continental island, the biota of which largely dates back to Gondwanan times owing to its geological origin and the presence of phylogenetic relicts. This view is contradicted by geological evidence indicating long Palaeocene and Eocene submersions and by recent biogeographic and phylogenetic studies, with molecular or geophysical dating placing the biota no older than the Oligocene. Phylogenetic relicts do not provide conclusive information in this respect, as their presence cannot be explained by simple hypotheses but requires assumption of many ad hoc extinction events. The implication of this new scenario is that all the New Caledonian biota colonized the island since 37 Ma Local richness can be explained by local radiation and adaptation after colonization but also by many dispersal events, often repeated within the same groups of organisms. Local microendemism is another remarkable feature of the biota. It seems to be related to recent speciation mediated by climate, orography, soil type and perhaps unbalanced biotic interactions created by colonization disharmonies. New Caledonia must be considered as a very old Darwinian island, a concept that offers many more fascinating opportunities of study.
Keywords: New Caledonia, biogeography, phylogenetics, endemism, dispersal, adaptation
1. Introduction
New Caledonia is a large and megadiverse tropical island in the southwest Pacific, with distinctive characteristics that make it a remarkable natural laboratory of evolution. Owing to its geological continental origin and the presence of apparent phylogenetic relicts, New Caledonia has long been considered a Gondwanan refuge where archaic groups have survived for 80 Ma (Holloway 1979; Morat 1993a). Reflecting this, the amazing New Caledonian species richness has been explained by local, long-term cladogenesis rather than rapid speciation after recent island colonization (e.g. Morat 1993b). This Gondwanan view became widespread during the past few decades (Lowry 1998; Lowry et al. 2005; Murienne et al. 2005) and has often been invoked as a reason to study the diverse New Caledonian biota (e.g. Mittermeier et al. 1996; Pagel 2003). New Caledonia was also characterized as a biodiversity hot spot owing to its high species richness and level of endemism, and the conservation issues raised by nickel mining, anthropogenic burning and forest logging (Bouchet et al. 1998; Myers et al. 2000), as well as the deleterious effects of invasive species (Gargominy et al. 1996; Jourdan et al. 2001; Keith 2005; Beauvais et al. 2006; Pascal et al. 2008). However, an alternative view, presented earlier but gaining far less traction, emphasized the absence of certain groups such as mammals and continental beetles and of clear geological evidence for Palaeocene marine transgression, and suggested that the biota was much more recent (Jeannel 1942; Faivre et al. 1955; Darlington 1957).
Convincing geological evidence now suggests that the second hypothesis is closer to the truth. New Caledonia's biodiversity is not that of a continental island that has retained many ancient groups since its separation from the northeastern margin of Australia, ca 80 Ma, but an oceanic island with a composite biota dominated by neoendemism and disharmonic colonization, a ‘Darwinian’ island (Gillespie & Roderick 2002). The question now for biologists is not so much whether the biota is Gondwanan and ancient but when and in what manner the modern biota assembled. Such questions can be addressed by modern phylogenetic approaches in the context of an accurate geological framework (Trewick et al. 2007). This is now possible for New Caledonia since the island has been the subject of increasing molecular phylogenetic and geological studies. We first briefly review the geological evidence that the biota is relatively young and then turn to the phylogenetic patterns recently deciphered in an effort to answer these questions.
2. Geological history and evolution of the biota
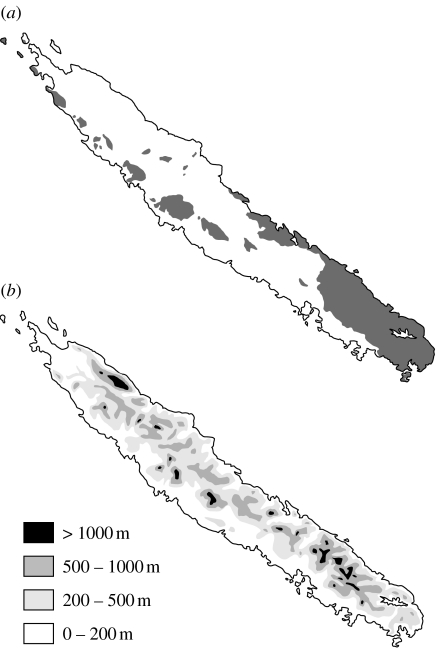
Oriented northwest to southeast roughly between latitudes 20 and 22° S, the island is 1220 km from Australia, 1700 km from New Zealand and approximately 400 km from the islands of Vanuatu. It is 16 890 km2 in area, with an elongate shape 500 km long and 50 km wide. Mountain ranges are complex and dissected by many rivers, with tablelands and peaks reaching elevations of more than 1600 m (figure 1). Most of the island is covered by wet evergreen forests with anthropogenic savannahs dominating at low elevations. A few small fragments of sclerophyll dry forest remain on the western coast and shrubby vegetation (‘maquis minier’) occurs on metalliferous soils, mostly in the south.
Figure 1.
(a) Distribution of ultramafic rocks (shaded areas) in New Caledonia. (b) Orography in New Caledonia showing several chains of mountains, peaking at more than 1600 m in the north and south.
New Caledonia formed from part of a continental fragment that began separating from Australia ca 83 Ma as the Tasman Sea began to form (Brothers & Lillie 1988; Neall & Trewick 2008). The New Caledonian area was subsequently subject to a series of dramatic geological events (Paris 1981a,b). In the Palaeocene, the part of Zealandia that subsequently became New Caledonia experienced a lengthy submersion in deep water, as evidenced by marine sedimentation (Globigerina limestone) and the formation of fine-grained black chert (‘phtanites’ of French authors), a type of rock indicating deep submersion as its structure is shaped by high pressure (Paris 1981a,b; Aitchison et al. 1998; Pelletier 2006). During the Eocene, the continental crust in the New Caledonia area was tectonically active, being in collision with the Loyalty Islands arc, and obduction at this time placed a layer of oceanic crust (lithosphere) over the submerged continental crust. New Caledonia emerged during an Oligocene lithosphere extension phase, uplifting with a cover of lithospheric ultramafic rocks (Paris 1981a,b; Aitchison et al. 1995; Cluzel et al. 2001; Crawford et al. 2003; Pelletier 2006; Schellart et al. 2006). The present-day mountains are relatively old since they are the product of complex orogenesis from Oligocene time, as indicated by several series of lateritic beds ranging from sea level to mountain tops (Chevillotte et al. 2006). In the period prior to subaerial emergence of New Caledonia, other islands might have existed on the Norfolk or the Loyalty Ridges but without any relationships or continuity with New Caledonia (Paris 1981a,b; Herzer et al. 1997; Meffre et al. 2006). Smaller islands such as Norfolk or the Loyalty Islands uplifted and emerged much later, 3.7 and 2 Ma as a result of volcanism and lithosphere flexure, respectively (Dubois et al. 1974).
These successive geological events have had important consequences for the evolution of the biota. First, more than 20 Ma of total submersion from the Palaeocene to the Eocene make it difficult to retain the notion that a Gondwanan biota has survived locally (Murienne et al. 2005; Murienne 2006; Pelletier 2006). Even if this biota persisted on emerged lands in the region, the occurrence of which is speculative, they had to disperse back to New Caledonia in the Oligocene. Second, ultramafic rocks obducted in the Eocene onto most of the New Caledonian mainland have given rise to an extensive area of metalliferous soils. Though subjected to several erosion cycles, they remain more extensive in the south. Being poor in nutrients and rich in metals (mainly nickel and copper), they are highly stressing substrates for many organisms and could have strongly constrained biotic evolution. Third, there is no evidence for direct exchange with New Zealand but only the possibility of stepping-stone dispersal after the Oligocene emersion, since part of the Norfolk Ridge was deep below sea level, deeper than the extent of major sea-level fluctuations. The terrestrial biota on neighbouring islands (Norfolk and the Loyalty Islands) is even more recent. Even though local volcanism has produced palaeo-islands there, reef structures and underlying layers indicate an unambiguous period of total submersion before their recent emersion (Dubois et al. 1974; Paris 1981a,b).
Owing to these three geological constraints, New Caledonia is a remarkable palaeogeographic model as it presents a combination of continental and oceanic features. In spite of a Gondwanan origin, it has undergone many recent tectonic events. Its elongated shape on the Norfolk Ridge made it roughly parallel to the subduction/obduction fronts that dramatically affected it, precluding a situation in which part of the island remained above the ocean surface while the rest was submerged. This strongly constrains biogeographic hypotheses, arguing for Oligocene recolonization after the very long Palaeocene and Eocene submersions. After emersion, its isolated position between two deep oceanic basins calls for rather simple explanations of its current biological diversity in terms of dispersal. In this geological context, the biota could be old, even though resulting from recolonization, and could have been shaped by orogenesis and extensive metalliferous soils over 37 Ma, a far longer time than on many other oceanic islands. Keeping in mind this remarkable geological framework, we next examine phylogenetic evidence separately to maintain logical independence between the two sources of evidence and to avoid circular reasoning, following the careful argument of Waters & Craw (2006).
3. Ancient radiations or repeated dispersal?
Answering this classical question is fundamental to understanding the evolution of biodiversity in New Caledonia and makes it possible to distinguish regional endemism (groups restricted to the New Caledonia mainland as a whole) from local endemism (groups restricted to certain locations in New Caledonia), an often confused issue for this island (Murienne 2006). Most studies citing the high rates of endemism (often close to 100%) in many groups of New Caledonian organisms actually refer to regional endemism examined in the context of large-scale phylogenetic studies (e.g. Morat et al. 1986; Chazeau 1993; Lowry 1998). Such studies often reveal that within certain New Caledonian groups, multiple species are nested within larger clades with taxa from Australia, New Zealand or New Guinea, calling for explanations in terms of recent dispersal. If, conversely, large New Caledonian clades are sister groups to taxa from other regions, dating the sister-group dichotomy is the only way to assess whether dispersal is again the explanation or if a vicariance hypothesis can be supported, even in the face of strong geological evidence to the contrary. Dating can be obtained from a molecular hypothesis with careful calibration of substitution rates or a geological hypothesis based on some physical measurements (e.g. K–Ar dating for some islands).
The clades that have been studied so far, however, do not show very clear-cut results in this respect. In several groups, there is evidence for multiple nested relationships involving taxa from New Caledonia and other regions, e.g. Nothofagus (Fagaceae; Swenson et al. 2001; Cook & Crisp 2005), Sapotaceae (Bartish et al. 2005) and Meryta (Araliaceae; Tronchet et al. 2005). Bartish et al. (2005) dated the oldest New Caledonian species in their study to 32.4 Ma with a molecular clock, corresponding to the Oligocene–Miocene transition.
Other groups show a single origin of their New Caledonian species. However, ages were not always evaluated by molecular dating in these studies but the geographical patterns implied are nonetheless very different from one another. In Araucaria (Araucariaceae), the New Caledonian radiation is sister to the species endemic to the much more recent Norfolk Island (Setoguchi et al. 1998). Implicitly, this New Caledonian radiation also has a recent origin (3.7 Ma). The same applies for Placostylus land snails, the sister group of which involves both the recently emerged Lord Howe Island and New Zealand (Trewick et al. in press). The New Caledonian sandalwood, Santalum austrocaledonicum, also differentiated recently, in this case from Vanuatu species 1–1.5 Ma (Harbaugh & Baldwin 2007). Other cases are different since the sister-group relationships involve large and remote regions such as Indo-Malaysia (eneopterine crickets: Desutter-Grandcolas & Robillard 2006; Robillard & Desutter-Grandcolas 2006), Australia and Africa (Proteaceae: Barker et al. 2007), or are poorly resolved (Araliaceae: Eibl et al. 2001), which leaves the dating question more open. For the Proteaceae, the dating (43–25 Ma) does not concur with a scenario of Gondwanan vicariance, which is otherwise supported by the tree topology (Barker et al. 2007). The New Caledonian freshwater shrimp genus Paratya (Page et al. 2005) and galaxiid fishes (Waters et al. 2000), sisters respectively to an Australian and a New Zealand group, are dated as younger than 12 and 9 Ma, respectively. The freshwater galaxiids, supposedly unable to disperse over the sea, were often considered a relict taxon, even though the occurrence of marine larvae is pervasive in this group (Waters et al. 2000).
Infrequent and distinct colonization events have been inferred in diving beetles (Balke et al. 2004, 2007a,b) and cockroaches (Murienne 2006; Murienne et al. in press a). In both, the occurrence of a few distinct clades in New Caledonia is an argument in favour of dispersal, at least for explaining the origin of one of the clades. In these cases, molecular dating indicates recent origins (14 and 9 Ma or 8.3 Ma, respectively).
We have not yet examined the case of the most remarkable supposedly relict groups, which are often referred to when arguing for a Gondwanan origin of the New Caledonian biota. New Caledonia harbours some taxa that are deeply embedded in the phylogenies of a number of large groups (but also lacks others such as Onychophora). The most famous is the endemic Amborella trichopoda, the sole member of the Amborellaceae and the sister group of all other flowering plants (Mathews & Donoghue 1999; Parkinson et al. 1999; Qiu et al. 1999; Soltis et al. 1999). Another remarkable example is the emblematic flightless bird, the kagu (Rhynochetos jubatus), the closest relatives of which occur in New Zealand and South America (Cracraft 2000; Fain & Houde 2004). A further example is the New Caledonian subfamily Troglosironidae (Opiliones), which is the sister group of taxa from South America and West Africa and only distantly related to Australian and the New Zealand taxa (Boyer et al. 2007). According to molecular dating, these Opiliones diversified recently in New Caledonia (28–49 Ma) even though the divergence from South America and West Africa is much older (124–221 Ma), the contrast between the two dates suggesting some extinction (Boyer et al. 2007). These deeply rooted and therefore relatively old groups, occurring in distant parts of the world, are frequently considered as relicts and used to support a Gondwanan origin for the biota of New Caledonia. Their presence in New Caledonia—considered as a geologically old land—is assumed to result from survival over 80 Ma. Contrary to this common reasoning, we submit that these groups do not provide much biogeographic and temporal information since their relatives are either absent from the region around New Caledonia or have a worldwide distribution. Their long time survival as relicts in New Caledonia is an indirect assumption requiring further assumption of many extinction events in neighbouring regions such as Australia or New Zealand (Grandcolas et al. in press).
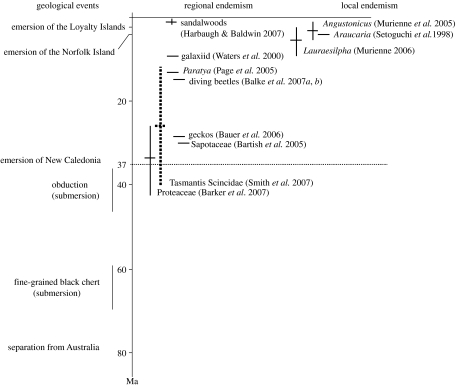
None of these studies provides clear evidence for old local diversification since most dates inferred from molecular phylogenies do not pre-date the Oligocene (figure 2). Several emblematic groups such as Araucaria and Nothofagus have even undergone more recent radiations or colonizations of New Caledonia. Also, there is ample evidence for the occurrence of repeated dispersal in many groups. Thus, there is no strong, unambiguous evidence for very old local Gondwanan radiations. Rather, many old Gondwanan groups are represented in New Caledonian by species of quite recent origin. As a whole, phylogenetic patterns and especially the dating are consistent with the geological framework presented above. Phylogenetic relicts remain puzzlingly enigmatic and their presence cannot be explained in a simple way.
Figure 2.
A time scale for the major geological events including the emersion of the New Caledonia mainland, 37 Ma ago (horizontal dotted line), and estimated ages (horizontal bars) for New Caledonian clades according to the studies cited (confidence intervals are shown if provided by the authors). Smith et al. (2007) provided a confidence interval for the age of scincid lizards of the whole of Tasmantis, indicated here with a vertical dotted line (the age of the New Caledonian clade itself was not provided but is necessarily more recent). Note that all these ages inferred independently from geology have been found to post-date the emersion of the island.
4. Local radiation and vacant niches
The question of radiation is often considered in relation to adaptation, and this is especially meaningful in the context of islands, where disharmony in colonization offers evolutionary opportunities for groups to diversify in ‘vacant’ niches (Losos et al. 1998; Gillespie 2004; Gillespie et al. 2008). Undoubtedly, some of the New Caledonian radiations have such adaptive backgrounds. The most remarkable examples are the monophyletic scincid and geckonid lizard radiations, the ecological diversity of which is unparalleled. These groups exhibit many remarkable foraging behaviours and morphologies (e.g. minute and giant species) on an island where other small vertebrates are scarce (Bauer & Sadlier 2000; Bauer et al. 2006; Smith et al. 2007). Another example is the radiation of the cricket genus Agnotecous (Eneopterinae), which includes many species with peculiar stridulatory apparatuses that emit songs in unusually high frequencies and with harmonic shifts (Robillard & Desutter-Grandcolas 2004; Robillard et al. 2007). The cockroach subfamily Tryonicinae (Blattidae) includes the speciose genus Lauraesilpha, the members of which exhibit a unique combination of behavioural traits, wood eating, intestinal ciliates and solitary habits (Grandcolas 1997; Murienne 2006; Murienne et al. in press a, submitted). These characteristics recall those of panesthiine cockroaches (Blaberidae), which include many wood-eating species in Australasia and throughout the southwest Pacific (including Vanuatu and Lord Howe Islands), but with the exception of New Caledonia (Roth 1991). The springtails of the genus Caledonimeria are among the largest species of Collembola (up to 8 mm), perhaps filling the niche of some other locally absent soil arthropods (D'Haese 2003).
Concerning adaptation and vacant niches, the expanding invasion by the little fire ant (Wasmannia auropunctata) is a sad natural experiment that demonstrates how the structure of native communities can evolve in a peculiar manner and offer evolutionary opportunities to colonizers on islands. Following its recent anthropogenic introduction, the little fire ant has colonized New Caledonian communities in which the local Pheidole ant species are unable to compete, unlike other Pheidole species in the native South American communities of W. auropunctata (Le Breton et al. 2005, 2007). The little fire ant seems to be preadapted to fill a vacant or weakly preoccupied niche in New Caledonia.
Every group studied has been characterized by some peculiar presumed adaptation, showing that evolutionary novelties are manifold in New Caledonia. What is now needed is to use new molecular phylogenetic hypotheses to document how such novelties appeared on the island. Did they occur repeatedly in New Caledonia by convergence with taxa absent from the island? If so, this would substantiate the assumption of evolutionary diversification into vacant niches.
5. Local endemism
Microendemism is extremely high on this medium-sized island (16 890 km2) and should not be neglected by emphasizing only larger-scale regional endemism (Murienne 2006). Along this 500 km long island, many related species are each often restricted to a very small area (often less than a few square kilometres). This amazing small-scale endemism has classically been explained by a combination of orography, soils and climates, diverse landscape features that result in a mosaic of habitats (Chazeau 1993; Morat 1993b). Many plant distributions are clearly related to these features, as many species are specialized for soils derived from either metamorphic–granitic, calcareous or ultramafic substrates. Plant species are also often limited to either dry sclerophyll forest, maquis shrubland or evergreen forest on different parts of the mountain ranges at different elevations, on the leeward, drier western coast or the windward, wetter eastern coast, from sea level to approximately 1600 m (Morat 1993b; Lowry 1998).
Until now, no attempt had been made to explain microendemism with reference to historical factors and speciation processes, except for some assumptions of climatic refuges for restricted distributions of palms (Pintaud & Jaffré 2001; Pintaud et al. 2001). However, recent phylogenetic studies provide some insight into microendemism patterns and their causes. Microendemism appears primarily related to allopatric speciation in plants (Eibl et al. 2001; Swenson et al. 2001, 2007; Bartish et al. 2005; Bottin et al. 2005; Tronchet et al. 2005), insects (Desutter-Grandcolas & Robillard 2006; Murienne et al. 2005, in press a), land snails (Trewick et al. in press) and lizards (Bauer & Sadlier 2000; Bauer et al. 2006; Smith et al. 2007), even though some cases of sympatry and even syntopy have also been documented in diving beetles (Balke et al. 2004, 2007b).
Most species in these various groups are restricted to a single mountain or group of mountains, sometimes in sympatry with species from more distantly related groups in the same clade. If the molecular substitution rates used by the authors of these studies are correctly calibrated, microendemism is a recent feature dating back only 2 or 3 Ma. This time frame is consistent with the most commonly accepted scenario in such studies that microendemic species have established through repeated vicariance events on neighbouring mountains subsequent to Quaternary climatic changes. The classical succession of dry/cold and hot/wet periods probably promoted allopatric speciation after range fragmentation as the altitudinal ranges of species on mountains changed. Sea-level changes during the same period could also have caused a few additional vicariance events between the most peripheral mountains connected by low passes to the main body of the central ranges. A stronger argument than one based on molecular clocks alone can be made when a clade of microendemic species in New Caledonia is sister to species occurring only on recently uplifted neighbouring islands (Norfolk, Lord Howe), which, as mentioned above, is the case for Araucaria trees (Setoguchi et al. 1998) and Placostylus land snails (Trewick et al. in press). Another case is the cockroach Angustonicus, two species of which occur only on the Loyalty Islands and are sister to all those on the New Caledonia mainland (Murienne et al. 2005). As argued by Murienne et al. (2005), this kind of relationship between taxa of the New Caledonia mainland and of a neighbouring and more recent island is good evidence for recent diversification in each group since sister groups are the same age, i.e. the age of the more recent island. Trewick (2000) reported a similar case for New Zealand and the Chatham Islands. A contrary interpretation would require invoking either unknown or extinct mainland species closely related to those on the more recent islands, a presumption that prevents any further logical biogeographic reasoning. However, following this presumption, some authors have hypothesized that palaeo-islands, pre-existing in the same place as recently uplifted islands (a geologically plausible assumption), could have harboured a member of the same clade, thus allowing for an older age (Heads 2005; Ladiges & Cantrill 2007). Such an assumption is, however, not warranted, as there is no actual evidence for recently uplifted islands occurring in conjunction with those palaeo-islands; and in any case, such a scenario would require several dispersal events among those islands in the past.
Based on these same studies, speciation seems to have occurred frequently between different mountains or mountain massifs but not necessarily along altitudinal gradients on a single mountain, even though some species are distributed preferentially at low or high elevations and some altitudinally vicariant distributions are known in plants (most summits are only 1000 m high, the highest being Mont Panié at 1628 m and Mont Humboldt at 1618 m). In this respect, there is no differentiation between low- and high-elevation populations of the same microendemic Lauraesilpha cockroach species, which are generally restricted to one mountain or massif (Murienne et al. in press a). Future studies will need to address this issue further by focusing on the highest summits to assess whether the presence or absence of altitudinal speciation in various groups could be related to the local size of the gradient.
Although adaptation appears to be a salient feature of regional endemism, this is not the case for local microendemism, in which many closely related and allopatrically distributed species apparently do not differ from each other in functional traits. For example, niche modelling among different species of the cockroach genera Angustonicus and Lauraesilpha failed to detect gross differences in microclimatic or altitudinal parameters (Murienne 2006; Murienne et al. in press b). Similarly, niches of Nothofagus species largely overlap with regard to climate even though they have different altitudinal distributions (Read et al. 2005). An exception to this pattern is the two ecologically segregated sympatric sister species of diving beetles on Mont Panié (Balke et al. 2007b). This probably means that microendemism and adaptation reflect complex evolutionary processes that take place at various levels in the phylogenetic hierarchy, with microendemism tending to happen more distally (and thus inferred to be more recent) and adaptive changes occurring more basally (and therefore regarded as more ancient).
Following the same adaptive line of reasoning, speciation related to soil diversity and especially to metalliferous soils has often been suggested to explain high local richness and persistence of adapted archaic groups when confronted with supposedly poorly adapted new immigrants (Holloway 1979; Morat 1993b; Haase & Bouchet 1998; Lowry 1998; Setoguchi et al. 1998; Bauer & Sadlier 2000). Metalliferous soils have been considered highly stressing substrates for many organisms, being poor in nutrients and rich in toxic metals including nickel (Proctor 2003). This opinion has been tempered by some recent studies showing that adaptation to metalliferous soils in plants is pervasive in many groups, being either symplesiomorphic or convergent (de Kok 2002). In insects, except for oligophagous or monophagous species feeding exclusively on hyperaccumulating plants (Boyd et al. 2006), diversification does not appear to be dependent on metalliferous soils (Desutter-Grandcolas & Robillard 2006; Murienne et al. submitted).
For all these reasons, a general explanation of New Caledonian microendemism fits better with a model of speciation involving niche conservatism and population divergence in environments isolated after climatic changes (e.g. mountains; Wiens 2004). New Caledonia is a good model for addressing such issues owing to its complex orography and elongate shape in a subtropical northwest–southwest geographical position resulting in climatic diversity and major effects of historical climate changes.
6. Conclusion and future research directions
Based on phylogenetic studies and geological evidence, New Caledonia must be regarded as a very old Darwinian island, dating to 37 Ma. The island has been subject to long periods of submersion in the Palaeocene–Eocene, the extent of which, contrary to the situation in New Zealand, is not disputed by geologists (Lee et al. 2001; Pole 2001; Trewick & Morgan-Richards 2005; Trewick et al. 2007). Consequently, the island's entire biota can only have resulted from a total recolonization since the Oligocene, which is consistent with independent dating from molecular phylogenetic studies.
In this context, New Caledonian diversity appears to have resulted from relatively ancient adaptive diversifications with abundant recent small-scale speciation involving niche conservatism. This contrasts with large tropical forest basins, where small-scale speciation plays a minor role (Moritz et al. 2000). A parallel conclusion was drawn by Latimer et al. (2005), who compared plant diversity in the South African fynbos with that in the Amazonian basin. In contrast to its role in the New Caledonian terrestrial biota, small-scale speciation also seems to play a minor role in New Caledonian marine ecosystems and especially on the sea mounts of the Norfolk Ridge, where diversity is more related to ecological patchiness than to microendemism on each individual sea mount (Samadi et al. 2006).
An increasing number of phylogenetic studies have made it possible to propose some preliminary general conclusions about the evolution of diversity in New Caledonia at both regional and local levels. Such studies should now be orientated to address several emerging questions. First, work on estimating the time of origin of groups that may represent pre-Oligocene relicts should be continued to confirm or falsify the Darwinian nature of New Caledonia. Second, fossil diversity for many groups, including insects, must be better studied, as it can shed light on past diversity and ecosystem history (Antoine et al. 2006). In this regard, some studies have revealed a recently extinct large vertebrate fauna, showing that generalizations based only on extant faunas can sometimes be misleading (Gaffney et al. 1984; Balouet & Buffetaut 1987; Balouet & Olson 1989). Third, efforts to understand the adaptive significance of New Caledonian evolutionary novelties must be sought by documenting whether taxa diversified into so-called vacant niches (Losos et al. 1998; Gillespie 2004). The amazingly small scale of speciation in New Caledonia is also an issue for study. In particular, modelling should address the question of whether landscape complexity combined with climatic changes is sufficient to explain the scale and amount of endemism. Biotic factors possibly promoting speciation also need to be considered, such as some remarkably low population densities, perhaps caused by high predation or competition pressures resulting from disharmonies in colonization.
Acknowledgments
We thank Raphaël Leblois, Sarah Samadi and Jérôme Sueur for reading the manuscript and provided insightful comments, and Pete Lowry who also corrected our English. This is a contribution from the project BIONEOCAL funded by the Agence Nationale de la Recherche (2008–2010) and funded previously through the Programme pluriformation ‘Structure et évolution des écosystèmes’ (Muséum national d'Histoire naturelle).
Footnotes
One contribution of 15 to a Theme Issue ‘Evolution on Pacific islands: Darwin's legacy’.
References
- Aitchison J.C, Clarke L, Meffre S, Cluzel D. Eocene arc-continent collision in New Caledonia and implications for regional southwest Pacific tectonic evolution. Geology. 1995;23:161–164. doi:10.1130/0091-7613(1995)023<0161:EACCIN>2.3.CO;2 [Google Scholar]
- Aitchison J.C, Ireland T.R, Clarke G.L, Cluzel D, Davis A.M, Meffre S. Regional implications of U/Pb SHRIMP age constraints on the tectonic evolution of New Caledonia. Tectonophysics. 1998;299:333–343. doi:10.1016/S0040-1951(98)00211-X [Google Scholar]
- Antoine P.O, et al. Amber from western Amazonia reveals Neotropical diversity during the Middle Miocene. Proc. Natl Acad. Sci. USA. 2006;103:13 595–13 600. doi: 10.1073/pnas.0605801103. doi:10.1073/pnas.0605801103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balke M, Ribera I, Vogler A.P. MtDNA phylogeny and biogeography of Copelatinae, a highly diverse group of tropical diving beetles (Dysticidae) Mol. Phylogenet. Evol. 2004;32:866–880. doi: 10.1016/j.ympev.2004.03.014. doi:10.1016/j.ympev.2004.03.014 [DOI] [PubMed] [Google Scholar]
- Balke M, Pons J, Ribera I, Sagata K, Vogler A.P. Infrequent and unidirectional colonization of hyperdiverse Papuadytes diving beetles in New Caledonia and New Guinea. Mol. Phylogenet. Evol. 2007a;42:505–516. doi: 10.1016/j.ympev.2006.07.019. doi:10.1016/j.ympev.2006.07.019 [DOI] [PubMed] [Google Scholar]
- Balke M, Wewalka G, Alarie Y, Ribera I. Molecular phylogeny of Pacific Island Colymbetinae: radiation of New Caledonian and Fijian species (Coleoptera, Dytiscidae) Zool. Scr. 2007b;36:119–227. doi:10.1111/j.1463-6409.2006.00265.x [Google Scholar]
- Balouet J.C, Buffetaut E. Mekosuchus inexpectatus, n.g., n.sp., crocodilien nouveau de l'Holocène de Nouvelle Calédonie. CR Acad. Sci. Sér. 2. 1987;304:853–856. [Google Scholar]
- Balouet J.C, Olson S.L. Fossil birds from late Quaternary deposits in New Caledonia. Smithson. Contrib. Zool. 1989;469:1–38. [Google Scholar]
- Barker N.P, Weston P.H, Rutschmann F, Sauquet H. Molecular dating of the ‘Gondwanan’ plant family Proteaceae is only partially congruent with the timing of the break-up of Gondwana. J. Biogeogr. 2007;34:2012–2027. doi:10.1111/j.1365-2699.2007.01749.x [Google Scholar]
- Bartish I.V, Swenson U.V, Munzinger J, Anderberg A.A. Phylogenetic relationships among New Caledonian Sapotaceae (Ericales): molecular evidence for generic polyphyly and repeated dispersal. Am. J. Bot. 2005;92:667–673. doi: 10.3732/ajb.92.4.667. doi:10.3732/ajb.92.4.667 [DOI] [PubMed] [Google Scholar]
- Bauer A.M, Sadlier R.A. Society for the Study of Amphibians and Reptiles; Ithaca, NY: 2000. The herpetofauna of New Caledonia. [Google Scholar]
- Bauer A.M, Jackman T, Sadlier R.A, Whitaker A.H. A revision of the Bavayia validiclavis group (Squamata: Gekkota: Diplodactylidae), a clade of New Caledonian geckos exhibiting microendemism. Proc. Calif. Acad. Sci. 2006;57:503–547. [Google Scholar]
- Beauvais, M. L., Coléno, A. & Jourdan, H. (eds) 2006 Les espèces envahissantes dans l'archiperl nèo-calèdonien. Invasive species in the New Caledonian archipelago Paris, France: IRD Editions.
- Bottin L, Verhaegen D, Tassin J, Olivieri I, Vaillant A, Bouvet J.M. Genetic diversity and population structure of an insular tree, Santalum austrocaledonicum in New Caledonian archipelago. Mol. Ecol. 2005;14:1979–1989. doi: 10.1111/j.1365-294X.2005.02576.x. doi:10.1111/j.1365-294X.2005.02576.x [DOI] [PubMed] [Google Scholar]
- Bouchet P, Jaffré T, Veillon J.-M. Threatened plants of New Caledonia: is the system of protected areas adequate? Biodivers. Conserv. 1998;7:109–135. [Google Scholar]
- Boyd R.S, Wall M.A, Jaffré T. Nickel levels in arthropods associated with Ni hyperaccumulator plants from an ultramafic site in New Caledonia. Insect Sci. 2006;13:271–277. doi:10.1111/j.1744-7917.2006.00094.x [Google Scholar]
- Boyer S.L, Clouse R.M, Benavides L.R, Sharma P, Schwendinger P.J, Karunarathna I, Giribet G. Biogeography of the world: a case study from cyphophthalmid Opiliones, a globally distributed group of arachnids. J. Biogeogr. 2007;34:2070–2085. doi:10.1111/j.1365-2699.2007.01755.x [Google Scholar]
- Brothers, R. N. & Lillie, A. R. 1988 Regional geology of New Caledonia. In The ocean basins and margins, vol. 7B (eds A. E. M. Nairn, F. G. Stehli & S. Uyeda), pp. 325–374. New York, NY: Plenum Press.
- Chazeau J. Research on New Caledonian terrestrial fauna: achievements and prospects. Biodivers. Lett. 1993;1:123–129. doi:10.2307/2999756 [Google Scholar]
- Chevillotte V, Chardon D, Beauvais A, Maurizot P, Colin F. Long-term tropical morphogenesis of New Caledonia (southwest Pacific): importance of positive epeirogeny and climate change. Geomorphology. 2006;81:361–375. doi:10.1016/j.geomorph.2006.04.020 [Google Scholar]
- Cluzel D, Aitchison J.C, Picard C. Tectonic accretion and underplating of mafic terranes in the Late Eocene intraoceanic fore-arc of New Caledonia (southwest Pacific): geodynamic implications. Tectonophysics. 2001;340:23–59. doi:10.1016/S0040-1951(01)00148-2 [Google Scholar]
- Cook L.G, Crisp M.D. Not so ancient: the extant crown group of Nothofagus represents a post-Gondwanan radiation. Proc. R. Soc. B. 2005;272:2535–2544. doi: 10.1098/rspb.2005.3219. doi:10.1098/rspb.2005.3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracraft J. Avian evolution, Gondwana biogeography and the Cretaceous–Tertiary mass extinction event. Proc. R. Soc. B. 2000;268:459–469. doi: 10.1098/rspb.2000.1368. doi:10.1098/rspb.2000.1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A.J, Meffre S, Symonds P.A. Chapter 25–120 to 0 Ma tectonic evolution of the southwest Pacific and analogous geological evolution of the 600 to 220 Ma Tasman Fold Belt system. Geol. Soc. Aust. Spec. Publ. 2003;22:377–397. [Google Scholar]
- Darlington P.J. Wiley; New York, NY: 1957. Zoogeography: the geographical distribution of animals. [Google Scholar]
- de Kok R. Are plant adaptations to growing on serpentine soil rare or common? A few case studies from New Caledonia. Adansonia. 2002;24:229–238. [Google Scholar]
- Desutter-Grandcolas L, Robillard T. Phylogenetic systematics and evolution of Agnotecous in New Caledonia (Orthoptera: Grylloidea, Eneopteridae) Syst. Entomol. 2006;31:65–92. doi:10.1111/j.1365-3113.2005.00299.x [Google Scholar]
- D'Haese C. Morphological appraisal of Collembola phylogeny with special emphasis on Poduromorpha and a test of the aquatic origin hypothesis. Zool. Scr. 2003;32:563–586. doi:10.1046/j.1463-6409.2003.00134.x [Google Scholar]
- Dubois J, Launay J, Recy J. Uplift movements in New Caledonia–Loyalty Islands area and their plate tectonics interpretation. Tectonophysics. 1974;24:133–150. doi:10.1016/0040-1951(74)90134-6 [Google Scholar]
- Eibl J.M, Plunkett G.M, Lowry P.P. Evolution of Polyscias sect. Tieghemopanax (Araliaceae) based on nuclear and chloroplast DNA sequence data. Adansonia. 2001;23:23–48. [Google Scholar]
- Fain G.M, Houde P. Parallel radiations in the primary clades of birds. Evolution. 2004;58:2558–2573. doi: 10.1111/j.0014-3820.2004.tb00884.x. doi:10.1554/04-235 [DOI] [PubMed] [Google Scholar]
- Faivre J.P, Poirier J, Routhier P. Nouvelles Editions Latines; Paris, France: 1955. Géographie de la Nouvelle-Calédonie. [Google Scholar]
- Gaffney E.S, Balouet J.C, de Froin F. New occurrences of extinct meiolaniid turtles in New Caledonia. Am. Mus. Novitates. 1984;2800:1–6. [Google Scholar]
- Gargominy O, Bouchet P, Pascal M, Jaffré T, Tourneur J.C. Conséquences des introductions d'espèces animales et végétales sur la biodiversité en Nouvelle-Calédonie. Rev. Ecol. (Terre Vie) 1996;51:375–401. [Google Scholar]
- Gillespie R.G. Community assembly through adaptive radiation in Hawaiian spiders. Science. 2004;303:356–359. doi: 10.1126/science.1091875. doi:10.1126/science.1091875 [DOI] [PubMed] [Google Scholar]
- Gillespie R.G, Roderick G.K. Arthropods on islands: colonization, speciation, and conservation. Annu. Rev. Entomol. 2002;47:595–632. doi: 10.1146/annurev.ento.47.091201.145244. doi:10.1146/annurev.ento.47.091201.145244 [DOI] [PubMed] [Google Scholar]
- Gillespie R.G, Claridge E.M, Roderick G.K. Biodiversity dynamics in isolated island communities: interaction between natural and human-mediated processes. Mol. Ecol. 2008;17:45–57. doi: 10.1111/j.1365-294X.2007.03466.x. doi:10.1111/j.1365-294X.2007.03466.x [DOI] [PubMed] [Google Scholar]
- Grandcolas, P. 1997 Systématique phylogénétique de la sous-famille des Tryonicinae (Dictyoptera, Blattaria, Blattidae). In Zoologia Neocaledonica, vol. 4 (eds J. Najt & L. Matile). Mém. Mus. nat. Hist. nat.171, 91–124.
- Grandcolas, P., Murienne, J., Desutter-Grandcolas, L., Robillard, T., Guilbert, E., D'Haese, C. & Deharveng, L. In press. Endemism in New Caledonia: the ghost of extinction past? Mem. Queensl. Mus
- Haase M, Bouchet P. Radiation of crenobiontic gastropods on an ancient continental island: the Hemistomia-clade in New Caledonia (Gastropoda: Hydrobiidae) Hydrobiologia. 1998;367:43–129. doi:10.1023/A:1003219931171 [Google Scholar]
- Harbaugh D.T, Baldwin B.G. Phylogeny and biogeography of the Sandalwoods (Santalum, Santalaceae): repeated dispersals throughout the Pacific. Am. J. Bot. 2007;94:1028–1040. doi: 10.3732/ajb.94.6.1028. doi:10.3732/ajb.94.6.1028 [DOI] [PubMed] [Google Scholar]
- Heads M. Dating nodes on molecular phylogenies: a critique of molecular biogeography. Cladistics. 2005;21:62–78. doi: 10.1111/j.1096-0031.2005.00052.x. doi:10.1111/j.1096-0031.2005.00078.x [DOI] [PubMed] [Google Scholar]
- Herzer R.H, et al. Seismic stratigraphy and structural history of the Reinga Basin and its margins, southern Norfolk Ridge system. NZ J. Geol. Geophys. 1997;40:425–451. [Google Scholar]
- Holloway, J. D. 1979 A survey of the Lepidoptera, biogeography and ecology of New Caledonia Series Entomologica, vol. 15. The Hague, Netherlands: Dr W. Junk.
- Jeannel R. Presses Universitaires de France; Paris, France: 1942. La genèse des faunes terrestres: éléments de biogéographie. [Google Scholar]
- Jourdan H, Sadlier R, Bauer A.M. Little fire ant invasion (Wasmannia auropunctata) as a threat to New Caledonian lizards: evidences from a sclerophyll forest (Hymenoptera: Formicidae) Sociobiology. 2001;38:283–301. [Google Scholar]
- Keith P. Revue des introductions de poissons et de Crustacés Décapodes d'eau douce en Nouvelle-Calédonie. Rev. Ecol. (Terre Vie) 2005;60:45–55. [Google Scholar]
- Ladiges P.Y, Cantrill D. New Caledonia-Australian connections: biogeographic patterns and geology. Aust. Syst. Bot. 2007;20:383–389. doi:10.1071/SB07018 [Google Scholar]
- Latimer A.M, Silander J.A, Cowling R.M. Neutral ecological theory reveals isolation and rapid speciation in a biodiversity hot spot. Science. 2005;309:1722–1725. doi: 10.1126/science.1115576. doi:10.1126/science.1115576 [DOI] [PubMed] [Google Scholar]
- Le Breton J, Jourdan H, Chazeau J, Orivel J, Dejean A. Niche opportunity and ant invasion: the case of Wasmannia auropunctata in a New Caledonian rain forest. J. Trop. Ecol. 2005;21:93–98. doi:10.1017/S0266467404002019 [Google Scholar]
- Le Breton J, Orivel J, Chazeau J, Dejean A. Unadapted behaviour of native, dominant ant species during the colonization of an aggressive, invasive ant. Ecol. Res. 2007;22:107–114. doi:10.1007/s11284-006-0014-z [Google Scholar]
- Lee D.E, Lee W.G, Mortimer N. Where and why have all the flowers gone? Depletion and turnover in the New Zealand Cenozoic angiosperm flora in relation to palaeogeography and climate. Aust. J. Bot. 2001;49:341–356. doi:10.1071/BT00031 [Google Scholar]
- Losos J.B, Jackman T.R, Larson A, de Queiroz K, Rodríguez-Schettino L. Contingency and determinism in replicated adaptive radiations of island lizards. Science. 1998;279:2115–2118. doi: 10.1126/science.279.5359.2115. doi:10.1126/science.279.5359.2115 [DOI] [PubMed] [Google Scholar]
- Lowry, P. P. 1998 Diversity, endemism, and extinction in the flora of New Caledonia: a review. In Proc. Int. Symp. on Rare, Threatened, and Endangered Floras of Asia and the Pacific (eds C. I. Peng & P. P. Lowry). Monograph Series, no. 16, pp. 181–206. Taipei, Taiwan: Institute of Botany, Academica Sinica.
- Lowry P.P, Munzinger J, Bouchet P, Géraux J, Bauer A.M, Langrand O, Mittermeier R.A. New Caledonia. In: Mittermeier R.A, Robles Gil P, Hoffmann M, Pilgrim J, Brooks T, Mittermeier C.G, Lamoreux J.L, da Fonseca G.A.B, editors. Hotspots revisited: earth's biologically richest and most threatened ecoregions. CEMEX; Mexico City, Mexico: 2005. pp. 193–197. [Google Scholar]
- Mathews S, Donoghue M.J. The root of angiosperm phylogeny inferred from duplicate phytochrome genes. Science. 1999;286:947–950. doi: 10.1126/science.286.5441.947. doi:10.1126/science.286.5441.947 [DOI] [PubMed] [Google Scholar]
- Meffre, S., Crawford, A. J. & Quilty, P. G. 2006 Arc-continent collision forming a large island between New Caledonia and New Zealand in the Oligocene. In Australian earth science convention (AESC), pp. 1–3. Melbourne, Australia: AESC.
- Mittermeier R.A, Werner T.B, Lees A. New Caledonia—a conservation imperative for an ancient land. Oryx. 1996;30:104–112. [Google Scholar]
- Morat P. Our knowledge of the flora of New Caledonia: endemism and diversity in relation to vegetation types and substrates. Biodivers. Lett. 1993a;1:72–81. doi:10.2307/2999750 [Google Scholar]
- Morat P. The terrestrial biota of New Caledonia. Biodivers. Lett. 1993b;1:69–71. doi:10.2307/2999749 [Google Scholar]
- Morat P, Veillon J.-M, MacKee H.S. Floristic relationships of New Caledonian rainforest phanerogams. Telopea. 1986;2:631–679. [Google Scholar]
- Moritz C, Patton J.L, Schneider C.J, Smith T.B. Diversification of rainforest faunas: an integrated molecular approach. Annu. Rev. Ecol. Syst. 2000;31:533–563. doi:10.1146/annurev.ecolsys.31.1.533 [Google Scholar]
- Murienne, J. 2006 Origine de la biodiversité en Nouvelle-Calédonie. Analyse phylogénétique de l'endémisme chez les insectes dictyoptères. Thèse de doctorat, Université Pierre et Marie Curie, Paris.
- Murienne J, Grandcolas P, Piulachs M.D, Bellés X, D'Haese C, Legendre F, Pellens R, Guilbert E. Evolution on a shaky piece of Gondwana: is local endemism recent in New Caledonia? Cladistics. 2005;21:2–7. doi: 10.1111/j.1096-0031.2004.00042.x. [DOI] [PubMed] [Google Scholar]
- Murienne, J., Pellens, R., Budinoff, R. B., Wheeler, W. C. & Grandcolas, P. In press a Phylogenetic analysis of the endemic to New Caledonia cockroach Lauraesilpha Testing competing hypotheses for the diversification. Cladistics24 (doi:10.1111/j.1096-0031.2008.00204.x)
- Murienne, J., Pellens, R. & Grandcolas, P. In press b Short-range endemism in New Caledonian insects: new species and distribution in the genus Lauraesilpha Grandcolas, 1997 (Insecta, Dictyoptera, Blattidae, Tryonicinae). In Zoologia Neocaledonica 6. Biodiversity studies in New Caledonia (ed. P. Grandcolas). Mém. Mus. nat. Hist. nat.196
- Murienne, J., D'Haese, C., Budinoff, R. B., Wheeler, W. C. & Grandcolas, P. Submitted. Molecular phylogenetic analysis of Blattidae (Hexapoda, Dictyoptera): evidence for a distinct and dual origin of regional endemism in New Caledonia.
- Myers N, Mittermeier R.A, Mittermeier C.G, da Fonseca G.A.B, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. doi:10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Neall V.E, Trewick S.A. The age and origin of the Pacific islands: a geological overview. Phil. Trans. R. Soc. B. 2008;363:3293–3308. doi: 10.1098/rstb.2008.0119. doi:10.1098/rstb.2008.0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page T.J, Baker A.M, Cook B.D, Hughes J.M. Historical transoceanic dispersal of a freshwater shrimp: the colonization of the South Pacific by the genus Paratya (Atyidae) J. Biogeogr. 2005;32:581–593. doi:10.1111/j.1365-2699.2004.01226.x [Google Scholar]
- Pagel C.N. The conifer flora of New Caledonia—stasis, evolution and survival in an ancient group. Acta Horticult. 2003;615:149–155. [Google Scholar]
- Paris, J. P. 1981a Géologie. In Atlas de le Nouvelle Calédonie et dépendances (ed. ORSTOM), pl. 9. Paris, France: Editions de l'Office de la Recherche Scientifique et Technique Outre-Mer.
- Paris J.P. Géologie de la Nouvelle-Calédonie. Un essai de synthése (Mémoire pour servir notice explicative à la carte géologique de la Nouvelle-Calédonie à l'échelle du 1/200000) Mém. Bureau Rech. Géol. Min. 1981b;113:1–278. [Google Scholar]
- Parkinson C.L, Adams K.L, Palmer J.D. Multigene analyses identify the three earliest lineages of extant flowering plants. Curr. Biol. 1999;9:1485–1488. doi: 10.1016/s0960-9822(00)80119-0. doi:10.1016/S0960-9822(00)80119-0 [DOI] [PubMed] [Google Scholar]
- Pascal M, Richer de Forges B, Le Guyader H, Simberloff D. Mining and other threats to the New Caledonia biodiversity hotspot. Conserv. Biol. 2008;22:498–499. doi: 10.1111/j.1523-1739.2008.00889.x. doi:10.1111/j.1523-1739.2008.00889.x [DOI] [PubMed] [Google Scholar]
- Pelletier, B. 2006 Geology of the New Caledonia region and its implications for the study of the New Caledonian biodiversity. In Compendium of marines species from New Caledonia, Forum BIOdiversité des Ecosystèmes Coralliens, 30 octobre–4 novembre 2006, Nouméa, Nouvelle-Calédonie (eds C. Payri & B. Richer de Forges). Documents Scientifiques et Techniques IRD, II 7, pp. 17–30. Nouméa, France: Institut de Recherche pour le Développement.
- Pintaud J.-C, Jaffré T. Patterns of diversity and endemism in palms on ultramafic rocks in New Caledonia. S. Afr. J. Sci. 2001;97:548–550. [Google Scholar]
- Pintaud J.-C, Jaffre T, Puig H. Chorology of New Caledonian palms and possible evidence of Pleistocene rain forest refugia. CR Acad. Sci. Ser. III. 2001;324:453–463. doi: 10.1016/s0764-4469(01)01312-9. doi:10.1016/S0764-4469(01)01312-9 [DOI] [PubMed] [Google Scholar]
- Pole M.S. Can long-distance dispersal be inferred from the New Zealand plant fossil record? Aust. J. Bot. 2001;49:357–366. doi:10.1071/BT00022 [Google Scholar]
- Proctor J. Vegetation and soil and plant chemistry on ultramafic rocks in the tropical Far East. Perspect. Plant Ecol. Evol. Syst. 2003;6:105–124. doi:10.1078/1433-8319-00045 [Google Scholar]
- Qiu Y.-L, et al. The earliest angiosperms: evidence from mitochondrial, plastid and nuclear genomes. Nature. 1999;402:404–407. doi: 10.1038/46536. doi:10.1038/46536 [DOI] [PubMed] [Google Scholar]
- Read J, Hope G.S, Hill R.S. Phytogeography and climate analysis of Nothofagus subgenus Brassospora in New Guinea and New Caledonia. Aust. J. Bot. 2005;53:297–312. doi:10.1071/BT04155 [Google Scholar]
- Robillard T, Desutter-Grandcolas L. High-frequency calling in Eneopterinae crickets (Orthoptera, Grylloidea, Eneopteridae): adaptive radiation revealed by phylogenetic analysis. Biol. J. Linn. Soc. 2004;83:577–584. doi:10.1111/j.1095-8312.2004.00417.x [Google Scholar]
- Robillard T, Desutter-Grandcolas L. Phylogeny of the cricket subfamily Eneopterinae (Orthoptera, Grylloidea, Eneopteridae) based on four molecular loci and morphology. Mol. Phylogenet. Evol. 2006;40:643–661. doi: 10.1016/j.ympev.2005.10.019. doi:10.1016/j.ympev.2005.10.019 [DOI] [PubMed] [Google Scholar]
- Robillard T, Desutter-Grandcolas L, Grandcolas P. A shift toward harmonics for high-frequency calling shown with phylogenetic study of frequency spectra in Eneopterinae crickets (Orthoptera, Grylloidea, Eneopteridae) Can. J. Zool. 2007;85:1264–1275. doi:10.1139/Z07-106 [Google Scholar]
- Roth, L. M. 1991 Blattodea. Blattaria (Cockroaches). In The insects of Australia. A textguide for students and researchers, vol. I (eds I. D. Naumann & CSIRO), pp. 320–329. Carlton, Australia: Melbourne University Press.
- Samadi S, Bottan L, Macpherson E, Richer de Forges B, Boisselier M.C. Seamount endemism questioned by the geographic distribution and population genetic structure of marine invertebrates. Mar. Biol. 2006;149:1463–1475. doi:10.1007/s00227-006-0306-4 [Google Scholar]
- Schellart W.P, Lister G.S, Toy V.G. A Late Cretaceous and Cenozoic reconstruction of the Southwest Pacific region: tectonics controlled by subduction and slab rollback processes. Earth-Sci. Rev. 2006;76:191–233. doi:10.1016/j.earscirev.2006.01.002 [Google Scholar]
- Setoguchi H, Osawa T.A, Pintaud J.C, Jaffré T, Veillon J.-M. Phylogenetic relationships within Araucariaceae based on RBCL gene sequences. Am. J. Bot. 1998;85:1507–1516. doi:10.2307/2446478 [PubMed] [Google Scholar]
- Smith S.A, Sadlier R.A, Bauer A.M, Austin C.C, Jackman T. Molecular phylogeny of the scincid lizards of New Caledonia and adjacent areas: evidence for a single origin of the endemic skinks of Tasmantis. Mol. Phylogenet. Evol. 2007;43:1151–1166. doi: 10.1016/j.ympev.2007.02.007. doi:10.1016/j.ympev.2007.02.007 [DOI] [PubMed] [Google Scholar]
- Soltis P.S, Soltis D.E, Chase M.W. Angiosperm phylogeny inferred from multiple genes as a research tool for comparative biology. Nature. 1999;402:402–404. doi: 10.1038/46528. doi:10.1038/46528 [DOI] [PubMed] [Google Scholar]
- Swenson U, Backlund A, McLoughlin S, Hill R.S. Nothofagus biogeography revisited with special emphasis on the enigmatic distribution of subgenus Brassospora in New Caledonia. Cladistics. 2001;17:28–47. doi:10.1111/j.1096-0031.2001.tb00109.x [Google Scholar]
- Swenson U, Munzinger J, Bartish I.V. Molecular phylogeny of Planchonella (Sapotaceae) and eight new species from New Caledonia. Taxon. 2007;56:329–354. [Google Scholar]
- Trewick S.A. Molecular evidence for dispersal rather than vicariance as the origin of flightless insect species on the Chatham Islands. NZ J. Biogeogr. 2000;27:1189–1200. doi:10.1046/j.1365-2699.2000.00492.x [Google Scholar]
- Trewick S.A, Morgan-Richards M. After the deluge: mitochondrial DNA indicates Miocene radiation and Pliocene adaptation of tree and giant weta (Orthoptera: Anostostomatidae) J. Biogeogr. 2005;32:295–309. doi:10.1111/j.1365-2699.2004.01179.x [Google Scholar]
- Trewick S.A, Paterson A.M, Campbell H.J. Hello New Zealand. J. Biogeogr. 2007;34:1–6. doi:10.1111/j.1365-2699.2006.01643.x [Google Scholar]
- Trewick, S. A., Brescia, F. & Jordan, C. In press. Diversity and phylogeny of New Caledonian Placostylus land snails; evidence from mitochondrial DNA. In Zoologia Neocaledonica 6. Biodiversity studies in New Caledonia (ed. P. Grandcolas). Mém. Mus. nat. Hist. nat.196
- Tronchet F, Plunkett G.M, Jeremie J, Lowry P.P. Monophyly and major clades of Meryta (Araliaceae) Syst. Bot. 2005;30:657–670. doi:10.1600/0363644054782279 [Google Scholar]
- Waters J.M, Craw D. Goodbye Gondwana? New Zealand biogeography, geology, and the problem of circularity. Syst. Biol. 2006;55:351–356. doi: 10.1080/10635150600681659. doi:10.1080/10635150600681659 [DOI] [PubMed] [Google Scholar]
- Waters J.M, Lopez J.A, Wallis G.P. Molecular phylogenetics and biogeography of galaxiid fishes (Osteichthyes: Galaxiidae): dispersal, vicariance, and the position of Lepidogalaxias salamandroides. Syst. Biol. 2000;49:777–795. doi: 10.1080/106351500750049824. doi:10.1080/106351500750049824 [DOI] [PubMed] [Google Scholar]
- Wiens J.J. Speciation and ecology revisited: phylogenetic niche conservatism and the origin of species. Evolution. 2004;58:193–197. doi: 10.1111/j.0014-3820.2004.tb01586.x. doi:10.1554/03-447 [DOI] [PubMed] [Google Scholar]