Abstract
Cypris larvae of barnacles are able to use a rapidly reversible temporary adhesion mechanism for exploring immersed surfaces. Despite decades of research interest, the means by which cyprids maintain attachment with surfaces prior to permanent settlement remain poorly understood. Here, we present novel observations on the morphology of ‘footprints’ of a putative adhesive secretion deposited by cyprids during surface exploration. Atomic force microscopy (AFM) was used to image footprints at high resolution and to acquire measurements of interaction forces. R–CH3- and R–NH2-terminated glass surfaces were used for comparison of footprint morphology, and it was noted that on R–NH2 each footprint comprised three times the volume of material deposited for footprints on R–CH3. Direct scaling of adhesion forces derived from AFM measurements did not adequately predict the real attachment tenacity of cyprids, and it is suggested that a mixture of ‘wet’ and ‘dry’ adhesive mechanisms may be at work in cyprid adhesion. High-resolution images of cyprid footprints are presented that correlate well with the known morphology of the attachment structures.
Keywords: atomic force microscopy, barnacle, cyprid, adhesion, biofouling
1. Importance of cyprids in marine fouling
The prevention and remediation of marine fouling is a multi-billion dollar (US$) industry (Yebra et al. 2004), and compositional or mechanical data pertaining to marine bioadhesives would be useful for hypothesis-driven coatings development. In fact, a considerable dearth of information remains regarding the composition of natural adhesives, with possible exceptions being the well-studied blue mussel (Mytilus edulis; Wiegemann 2005; Lee et al. 2006; Aldred et al. 2006, 2007; Aldred 2007) and barnacle adult cement system (Kamino 2006). Barnacles are important marine fouling organisms due to their large size, hard, calcareous body form (Anderson 2003) and generally gregarious nature (Crisp & Meadows 1962). Barnacle larvae settle readily on man-made structures, resulting in increased hydrodynamic drag and damage to protective coatings (Christie & Dalley 1987). Specifically, the prominence of barnacles on ship hulls has directed research towards strategies designed to prevent settlement or attachment of these organisms.
2. Barnacle cyprid temporary adhesion
Temporary adhesion is fundamental to barnacles. Without it, settlement and subsequent development and growth to the adult barnacle could not occur. Preventing cyprid attachment is thus a logical point of attack for fouling control.
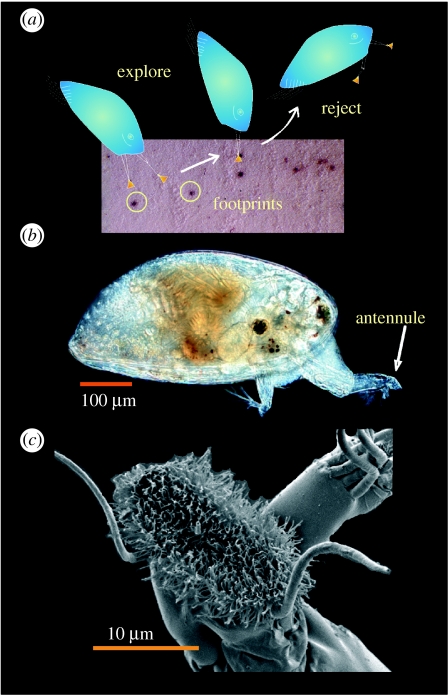
The barnacle cypris larva (figure 1a,b (N.B. Balanus amphitrite)) has two discrete adhesion systems, one temporary and one permanent (Phang et al. 2006), both unrelated to the well-studied adult cement system. Prior to permanent attachment, cyprids explore surfaces using a form of bipedal ‘walking’ (electronic supplementary material, movie S1). It has been suggested that cyprid temporary adhesion is facilitated by a glycoproteinaceous secretion derived from modified hypodermal glands within their paired antennules (Nott & Foster 1969; Walker & Yule 1984); this secretion is expressed externally onto the antennular attachment disc (figure 1c). During surface exploration, ‘footprints’ are deposited by cyprids serving as a settlement cue for subsequently exploring larvae (figure 1a; Clare & Nott 1994; Matsumura et al. 1998; Dreanno et al. 2006a,b). Although the antennular secretion (syn. footprint material) is often referred to in the literature as ‘temporary adhesive’, it is unclear to what degree the antennular secretion acts as a ‘wet’ adhesive. Its role may be more akin to a water displacer or release agent. Adhesion through intimate contact of the cuticular villi (on the base of the adhesive disc; figure 1c) with a surface could, theoretically, operate underwater as it does in air for some arthropods and reptiles (Autumn et al. 2000; Niederegger et al. 2002; Arzt et al. 2003; Autumn 2006; Federle 2006). This direct adhesive interaction between two solids is often referred to as ‘dry adhesion’. By comparison, ‘wet adhesion’ is used to describe two solids that are held together by a viscous mechanism—usually the presence of a liquid adhesive.
Figure 1.
The ultrastructure and morphology of the cyprid temporary adhesive system. (a) A schematic of cyprid surface exploration on nitrocellulose membrane with footprints made visible by immunostaining. (b) A light micrograph of a Balanus amphitrite cyprid (similar, but larger, cyprids of Semibalanus balanoides were used in experiments). (c) The ultrastucture of the antennular attachment disc from B. amphitrite.
3. Atomic force microscopy
For two decades, researchers have attempted to isolate cyprid footprints in a form that would permit investigation by modern microscopy and proteomics techniques without any notable success. Therefore, few publications exist on the topic (e.g. Walker & Yule 1984). The bottleneck in this research has been procuring sufficient material for study as the volume of a cyprid footprint is of the order of nanolitres. Atomic force microscopy (AFM) is particularly appropriate for this type of work since it allows simultaneous imaging with measurement of interaction forces between the AFM cantilever and the subject material. Location of cyprid footprints that are optically transparent and measure only approximately 50 μm (Semibalanus balanoides) is another obstacle that has, thus far, hindered their study. Nitrocellulose membrane retains cyprid footprints (figure 1a; for methods see Matsumura et al. (1998)), but is not a suitable substratum for AFM due to its relatively large topography. Smooth materials, such as clean glass, generally do not retain footprints well. In R–NH2-terminated glass, we identified a surface that both retains footprints reliably and is smooth enough to allow study by AFM at high resolution. For comparisons of footprint morphology, R–CH3-terminated glass has also been included here, where footprints were far less abundant. It is unclear at this stage whether the dichotomy in apparent footprint retention between these two functionalities is due to differences in their reactivity with the footprint material, or alternatively due to ‘intentional’ differences in footprint deposition by cyprids on different surfaces.
Cyprids of the acorn barnacle (S. balanoides) were collected by plankton tow at Cullercoats, UK (55.1° N, 1.26° W) during April 2006. They were stored (1 cyprid ml−1) in 2 l glass beakers containing 33 PSU artificial seawater (ASW; Tropic Marin) at 6°C prior to use. Cyprids were introduced into a dish containing R–NH2 (θadv=60°, θrec=25°) or R–CH3 (θadv=85°, θrec=55°)-terminated glass (obtained by gas-phase evaporation of 3-aminopropyltriethoxysilane or dodecyltriethoxysilane from Sigma Aldrich) and would typically attach and begin to explore when stimulated by water currents produced with a Pasteur pipette. Explored areas of the glass were marked underneath and cyprids were then removed from the dish. Surfaces were flushed with filtered ASW to minimize contamination and Petri dishes were transferred to the Dimension D3100 AFM (Veeco/Digital Instruments (DI), Santa Barbara, CA). The search for footprints was focused on the marked regions.
Although footprints deposited onto the different surfaces had similar areas (table 1), the thickness and, therefore, volumes of material comprising footprints on the R–NH2 surface were over three times that of footprints on R–CH3. Assuming finite production, Walker & Yule (1984) estimated that the hypodermal glands responsible for ‘adhesive’ synthesis could produce up to 1.9×104 μm3 of the material. Using the present footprint volume data, a finite resource would limit the number of footprints to 530±20 on R–NH2 glass and 1600±40 on R–CH3 glass for a single S. balanoides cyprid. This corresponds to a distance traversed between 35 and 110 cm, respectively, using a measured individual pace distance of 660 μm.
Table 1.
Information regarding cyprid footprints and their adhesive strength calculated from AFM measurements of the footprint material.
| glass surface functionalization | mean footprint area (μm2±s.d.) | r.m.s. thickness (nm±s.d.) | volume (μm3±s.d.) | estimated exploration steps | estimated mean pull-off force (nN±s.d.) | estimated protein adhesion strength (MPa) |
|---|---|---|---|---|---|---|
| NH2 (θadv=60°) | 1928±259 | 18.9±3.0 | 36.7±10.7 | 535 | 0.41±0.2 | 0.026–0.038 |
| CH3 (θadv=85°) | 2157±201 | 5.3±1.0 | 11.4±1.1 | 1663 | — | — |
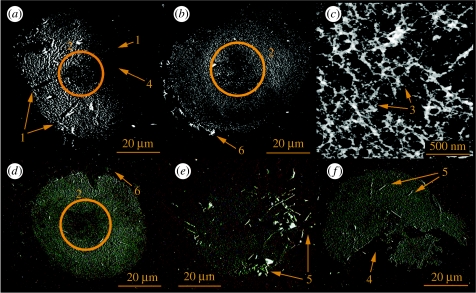
Cyprids are highly discriminating about the surfaces they permanently attach to. Sensation of surface character is likely to occur throughout exploration by cyprids, allowing informed ‘decisions’ to be made regarding surface selection. It appears that either the radial sense organs of the third antennular segment or postaxial seta III (Nott & Foster 1969; figure 1c) often curl beneath the attachment disc during exploration leaving their impression in the footprint residue (figure 2a (1)). The axial sense organ in the centre of the adhesive disc also appears to be accommodated by a void in the centre of the footprint where little or no material is present (figure 2a,b,d (2)).
Figure 2.
(a–f) AFMs of replicate S. balanoides footprints demonstrating a variety of traits, namely (1) the presence of sensory setae beneath the attachment disc during exploration is suggested by their negative imprints in the deposited material; (2) the centre of the attachment disc, where the axial sense organ is located, either does not secrete any footprint material or is never in direct contact with the surface; (3) the fibrillar/porous nature of the footprint material, perhaps resulting from the presence of cuticular villi in the adhesive deposit; (4) peeling of the footprint material from the surface; (5) bacteria associated with cyprid footprints and (6) the contact point of the cuticular velum that encircles the cyprid attachment disc.
The deposited footprint material is fibrillar in appearance and nanofibrils varied in height between 7 and 150 nm. Figure 2c (3) is a high-resolution AFM image of unmodified, hydrated footprint material on R–NH2. Diagnostic ‘fingerprint’ signatures of self-assembled adhesive nanofibres have been observed by AFM force spectroscopy in the mucilage of diatoms and the terrestrial alga Prasiola linearis (Higgins et al. 2003; Dugdale et al. 2005, 2006; Mostaert et al. 2006). These adhesive proteins are able to form web-like networks to provide mechanical toughness which enhances the adhesive's ability to resist deformation under shear forces (Smith et al. 1999).
The antennular secretion, it seemed, had a stronger affinity to R–NH2 surfaces than R–CH3, resulting in more material remaining on those surfaces after each step. In addition, if the interfacial energies in the footprint/glass/antennular disc system govern the frequency with which footprints remained on the test surfaces, the larger number of footprints observed on R–NH2 would anecdotally suggest higher adherence on that surface. When the adhesive joint between an antennule and R–NH2 was broken, the footprint material remained on the surface more often than when the same process occurred on R–CH3 and also showed evidence of peeling on R–CH3 (figure 2a,f (4)), where some material had clearly remained on the antennular disc. This system is probably governed by conventional wetting theory. Therefore, on failure of the adhesive joint between the antennule and the substratum, whether water enters between the surface and the glycoprotein or between the glycoprotein and antennular disc/cuticular villi depends on their respective work of adhesion (Aldred 2007). After a matter of hours, footprints began to attract bacteria that were, ostensibly, capable of digesting the footprint material (figure 2e,f (5)).
4. Possible modes of adhesion
A visco-adhesive mechanism, with the antennular secretion as an adhesive, became the generally accepted theory regarding cyprid temporary adhesion during the 1980s and persisted, primarily through the exclusion of other possibilities (Yule & Walker 1987). Empirical estimates of adhesion, which were not made until the mid-1980s (Yule & Crisp 1983; Yule & Walker 1984, 1985, 1987), demonstrated the strength of S. balanoides temporary adhesion to be of the order of 0.068–0.076 MPa on clean glass. The absence of appropriate musculature within the third antennular segment (Nott & Foster 1969) and the observation that cyprids can effectively adhere using only 50% of their attachment disc surface ultimately precluded the suggestion (Lindner 1984) that cyprids attach purely through suction—facilitated by the cuticular velum that encircles the attachment disc. There is evidence here, however, that this velum at least makes contact with the surface, and its contact point could be seen as a ‘halo’ of material around the footprint (figure 2b,d (6)).
We sought to validate the observations of Yule & Crisp (1983) by measuring the interaction forces between an AFM tip and the footprint material and scaling up to the size of two footprints; if the glycoproteinaceous secretion alone mediates attachment, then estimate and empirical measurement should return similar values. Footprint protein pull-off forces were obtained from 2500 force–distance curves and produced a mean force of 0.41±0.20 nN s.d. This force was scaled from an AFM tip, with apex diameter 100 nm, to the area of two antennular discs. Furthermore, it was assumed that the presence of cuticular villi between the antennular disc and the surface would impart an estimated 50% porosity to the footprint, resulting in the fibrillar appearance of footprints at high resolution (figure 2c (3)). From this calculation, cyprids could theoretically attach with a tenacity of 0.026 MPa (table 1), one-third of the empirical value (0.068–0.076 MPa on glass; Yule & Walker 1984). Adhesion of the footprint material to silicone nitrile AFM tips would differ from that on glass, although it is unclear whether this difference would be enough to account for the threefold discrepancy between empirical measurement and AFM estimate in this case.
The presence of cuticular villi on the base of the antennular disc may serve to enhance or perturb adhesion between the antennule and the footprint material. Their inclusion in the above calculation as simply introducing porosity into the adhesive may not, therefore, be entirely accurate. Furthermore, there is the possibility that if the villi totally penetrate the footprint material, they may contact the surface and act in a similar way to the pulvilli of flies or spatulae of geckos (Huber et al. 2005), enhancing adhesion through contact splitting. From this perspective, the secreted material could displace water, providing an environment more conducive to intermolecular/electrostatic adhesion with a lower dielectric constant.
5. Summary
Barnacle cyprids have evolved a method of attaching rapidly and reversibly to almost any immersed solid object, although exactly how they do so remains a mystery. The authors do not accept that the cuticular villi, with their diverse morphology between genera of barnacles, serve only as a retention mechanism for footprint material on the antennule (Moyse et al. 1985). If the adhesive disc villi of cyprids are shown to contribute to adhesion through surface interaction, it would be the first reported instance of such a mechanism being used by an organism underwater. Valuable insight into the mechanism of cyprid temporary adhesion has been presented here, and it is hoped that future work will build on these observations to further understand this intriguing and complex system.
Acknowledgments
We thank Dr Nikodem Tomczak for providing software for force curve analysis, and Xing Yi Ling, Dr Holger Schönherr, Dr Juang-Horng Chong and Dr Tony Khor for critical reading of the manuscript. This work was supported by a Dutch Polymer Institute grant no. DPI-510 (to G.J.V.); a Natural Environment Research Council 350 (NERC) studentship (NER/S/A/2002/10449, to N.A.) and a US Office of Naval Research grant (N00014-05-1-0767, to A.S.C.); I.Y.P. thanks SYNTHESYS for funding work with Prof. Jens Hoeg at the University of Copenhagen.
Supplementary Material
A short movie of a B. amphitrite cyprid exploring over a glass microscope slide
References
- Aldred, N. 2007 The adhesion and adhesives of barnacles (Balanus amphitrite; Semibalanus balanoides) and mussels (Mytilus edulis), pp. 201. PhD thesis, Newcastle University.
- Aldred N, Ista L.K, Callow M.E, Callow J.A, Lopez G.P, Clare A.S. Mussel (Mytilus edulis) byssus deposition in response to variations in surface wettability. J. R. Soc. Interface. 2006;3:37–43. doi: 10.1098/rsif.2005.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldred N, Wills T, Williams D.N, Clare A.S. Tensile and dynamic mechanical analysis of the distal portion of mussel (Mytilus edulis) byssal threads. J. R. Soc. Interface. 2007;4:1159–1167. doi: 10.1098/rsif.2007.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D.T. Chapman and Hall; London, UK: 2003. Barnacles: structure, function, development and evolution. [Google Scholar]
- Arzt E, Gorb S, Spolenak R. From micro to nano contacts in biological attachment devices. Proc. Natl Acad. Sci. USA. 2003;100:10 603–10 606. doi: 10.1073/pnas.1534701100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autumn K. Properties, principles, and parameters of the gecko adhesive system. In: Smith A.M, Callow J.A, editors. Biological adhesives. Springer; Heidelberg, Germany: 2006. pp. 225–256. [Google Scholar]
- Autumn K, Liang Y.A, Hsieh S.T, Zesch W, Chan W.P, Kenny T.W, Fearing R, Full R.J. Adhesive force of a single gecko foot-hair. Nature. 2000;405:681–685. doi: 10.1038/35015073. [DOI] [PubMed] [Google Scholar]
- Christie A.O, Dalley R. Barnacle fouling and its prevention. In: Southward A.J, editor. Crustacean issues 5: barnacle biology. A. A. Balkema; Rotterdam, The Netherlands: 1987. pp. 419–433. [Google Scholar]
- Clare A.S, Nott J.A. Scanning electron microscopy of the 4th antennular segment of Balanus amphitrite amphitrite. J. Mar. Biol. Assoc. UK. 1994;74:967–970. [Google Scholar]
- Crisp D.J, Meadows P.S. Chemical basis of gregariousness in cirripedes. Proc. R. Soc. B. 1962;156:500–520. doi: 10.1098/rspb.1962.0052. [DOI] [Google Scholar]
- Dreanno C, Kirby R.R, Clare A.S. Smelly feet are not always a bad thing: the relationship between cyprid footprint protein and the barnacle settlement pheromone. Biol. Lett. 2006a;2:423–425. doi: 10.1098/rsbl.2006.0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreanno C, Matsumura K, Dohmae N, Takio K, Hirota H, Kirby R.R, Clare A.S. An alpha(2)-macroglobulin-like protein is the cue to gregarious settlement of the barnacle Balanus amphitrite. Proc. Natl Acad. Sci. USA. 2006b;103:14 396–14 401. doi: 10.1073/pnas.0602763103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugdale T.M, Dagastine R, Chiovitti A, Mulvaney P, Wetherbee R. Single adhesive nanofibers from a live diatom have the signature fingerprint of modular proteins. Biophys. J. 2005;89:4252–4260. doi: 10.1529/biophysj.105.062489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugdale T.M, Dagastine R, Chiovitti A, Wetherbee R. Diatom adhesive mucilage contains distinct supramolecular assemblies of a single modular protein. Biophys. J. 2006;90:2987–2993. doi: 10.1529/biophysj.105.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federle W. Why are so many adhesive pads hairy? J. Exp. Biol. 2006;209:2611–2621. doi: 10.1242/jeb.02323. [DOI] [PubMed] [Google Scholar]
- Higgins M.J, Molino P, Mulvaney P, Wetherbee R. The structure and nanomechanical properties of the adhesive mucilage that mediates diatom–substratum adhesion and motility. J. Phycol. 2003;39:1181–1193. doi: 10.1111/j.0022-3646.2003.03-027.x. [DOI] [Google Scholar]
- Huber G, Mantz H, Spolenak R, Mecke K, Jacobs K, Gorb S.N, Arzt E. Evidence for capillarity contributions to gecko adhesion from single spatula nanomechanical measurements. Proc. Natl Acad. Sci. USA. 2005;102:16 293–16 296. doi: 10.1073/pnas.0506328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamino K. Barnacle underwater attachment. In: Smith A.M, Callow J.A, editors. Biological adhesives. Springer; Heidelberg, Germany: 2006. [Google Scholar]
- Lee H, Scherer N.F, Messersmith P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl Acad. Sci. USA. 2006;103:12 999–13 003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner E. The attachment of macrofouling invertebrates. In: Costlow J.D, Tipper R.C, editors. Marine biodeterioration: an interdisciplinary study. Spon; London, UK: 1984. pp. 183–202. [Google Scholar]
- Matsumura K, Nagano M, Kato-Yoshinaga Y, Yamazaki M, Clare A.S, Fusetani N. Immunological studies on the settlement-inducing protein complex (SIPC) of the barnacle Balanus amphitrite and its possible involvement in larva–larva interactions. Proc. R. Soc. B. 1998;265:1825–1830. doi: 10.1098/rspb.1998.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostaert A.S, Higgins M.J, Fukuma T, Rindi F, Jarvis S.P. Nanoscale mechanical characterisation of amyloid fibrils discovered in a natural adhesive. J. Biol. Phys. 2006;32:393–401. doi: 10.1007/s10867-006-9023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyse J, Jensen P.G, Høeg J.T, Al-Yahya H. Attachment organs in cypris larvae: using scanning electron microscopy. In: Schram F.R, Høeg J.T, editors. Crustacean issues 10: new frontiers in barnacle evolution. A. A. Balkema; Rotterdam, The Netherlands: 1985. pp. 153–178. [Google Scholar]
- Niederegger S, Gorb S, Jiao Y.K. Contact behaviour of tenent setae in attachment pads of the blowfly Calliphora vicina (Diptera, Calliphoridae) J. Comp. Physiol. A. 2002;187:961–970. doi: 10.1007/s00359-001-0265-7. [DOI] [PubMed] [Google Scholar]
- Nott J.A, Foster B.A. On the structure of the antennular attachment organ of cypris larvae of Balanus balanoides (L) Phil. Trans. R. Soc. B. 1969;256:115–134. doi: 10.1098/rstb.1969.0038. [DOI] [Google Scholar]
- Phang I.Y, Aldred N, Clare A.S, Callow J.A, Vancso G.J. An in situ study of the nanomechanical properties of barnacle (Balanus amphitrite) cyprid cement using atomic force microscopy (AFM) Biofouling. 2006;22:245–250. doi: 10.1080/08927010600857686. [DOI] [PubMed] [Google Scholar]
- Smith B.L, et al. Molecular mechanistic origin of the toughness of natural adhesives, fibres and composites. Nature. 1999;399:761–763. doi: 10.1038/21607. [DOI] [Google Scholar]
- Walker G, Yule A.B. Temporary adhesion of the barnacle cyprid: the existence of an antennular adhesive secretion. J. Mar. Biol. Assoc. UK. 1984;64:679–686. [Google Scholar]
- Wiegemann M. Adhesion in blue mussels (Mytilus edulis) and barnacles (genus Balanus): mechanisms and technical applications. Aquat. Sci. 2005;67:166–176. doi: 10.1007/s00027-005-0758-5. [DOI] [Google Scholar]
- Yebra D.M, Kiil S, Dam-Johansen K. Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004;50:75–104. doi: 10.1016/j.porgcoat.2003.06.001. [DOI] [Google Scholar]
- Yule A.B, Crisp D.J. Adhesion of cypris larvae of the barnacle, Balanus balanoides, to clean and arthropodin treated surfaces. J. Mar. Biol. Assoc. UK. 1983;63:261–271. [Google Scholar]
- Yule A.B, Walker G. The temporary adhesion of barnacle cyprids: effects of some differing surface characteristics. J. Mar. Biol. Assoc. UK. 1984;64:429–439. [Google Scholar]
- Yule A.B, Walker G. Settlement of Balanus balanoides: the effect of cyprid antennular secretion. J. Mar. Biol. Assoc. UK. 1985;65:707–712. [Google Scholar]
- Yule A.B, Walker G. Adhesion in barnacles. In: Southward A.J, editor. Crustacean issues 5: barnacle biology. A. A. Balkema; Rotterdam, The Netherlands: 1987. pp. 389–402. [Google Scholar]
Notice of correction
Figure 2c is now correct. 15 November 2007
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A short movie of a B. amphitrite cyprid exploring over a glass microscope slide