Abstract
Glue-free reversible adhesion was achieved underwater using a beetle-inspired mushroom-shaped fibrillar microstructure. Structured surfaces reveal a 25% increase in pull-off force when immersed in water and their underwater attachment is 20 times more effective than that of flat surfaces. The van der Waals interaction that underlies the adhesion of the mushroom-shaped fibrillar microstructure is significantly enhanced by a suction effect when underwater. This results in a higher adhesive capability of the material, with potential in medicine, bio- and marine technologies and a range of applications in liquid-dominated environments.
Keywords: biomimetics, surface patterning, polymer, adhesion, underwater
1. Introduction
Underwater adhesion is greatly complicated by the difficulty in displacing water from the adhesive interface and water's ability to weaken many forms of chemical bonds (Smith & Callow 2006). Nevertheless, adhesion is a way of life in aquatic environments, and nature provides many examples of both permanent and temporary underwater attachment based on complex polymer glues (Waite 1987; Flammang 1996; Smith & Callow 2006). In terrestrial environments, many organisms have developed efficient fibrillar adhesive mechanisms based on contact splitting (Arzt et al. 2003), which allow them to benefit from secretion-mediated capillary attractive forces and van der Waals interactions (Stork 1980a; Autumn et al. 2000, 2002). Here, we report that the idea of contact splitting can also be adapted for the generation of glue-free reversible underwater adhesion.
Following the progress made in understanding the mechanism behind the ability of some terrestrial animals to attach dynamically to uneven surfaces (Autumn et al. 2000, 2002; Beutel & Gorb 2001), it was experimentally confirmed that adhesion-oriented geometry of biological fibrillar attachment systems may be employed to manufacture artificial, self-cleaning, re-attachable dry adhesives (Geim et al. 2003). However, only recently, an effective biomimetic fibrillar adhesive microstructure was produced (Gorb et al. 2007). This surface microstructure is based on the analysis of the functional morphology of the tarsal hairs found in numerous species of beetles from the family Chrysomelidae (Stork 1980b; Gorb 2001) and is defined by mushroom-shaped elastic microfibres covering the surface. Considering that intermolecular van der Waals forces should act across different media, we examined this beetle-inspired dry adhesive to determine the extent to which contact splitting affects adhesion underwater.
2. Experimental details
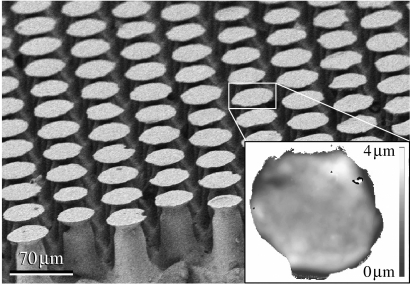
Figure 1 shows our beetle-inspired fibrillar adhesive microstructure made of polyvinylsiloxane (PVS). The structured surface (Gottlieb Binder GmbH, Holzgerlingen, Germany) consisted of hexagonally distributed mushroom-shaped microfibres of approximately 100 μm in height, bearing terminal contact plates of 48 μm in mean diameter. The mean area density of the terminal contact plates was 43%. Four structured and four flat PVS samples were tested in contact with hydrophilic and hydrophobic (silanized) flat glass substrates (water contact angle of 7° and 110°, respectively) in ambient air at 25% relative humidity, and in de-ionized water. Each of the 32 tests was repeated at least four times. For the underwater experiments, the PVS sample and glass substrate were submerged entirely in water. The rourhness average (Ra) of the PVS and glass surface was approximately 1 and 85 nm, respectively. Each test consisted of preloading a PVS disc sample 1 mm in height and 2 mm in diameter with 90 mN, and then determining the pull-off force while withdrawing the glass substrate from the contact at a velocity of 100 μm s−1. This was done with a home-made microtribometer (Varenberg & Gorb 2007) using a self-aligning system of sample holders to measure contact forces in a flat-on-flat contact scheme.
Figure 1.
Beetle-inspired adhesive microstructure. Scanning electron micrograph presents mushroom-shaped microfibres near the edge of a structured sample, illustrating the two-level hierarchy of terminal contact plates and stalks of the microfibres. Inset displays the height image of a single terminal contact plate.
3. Results and discussion
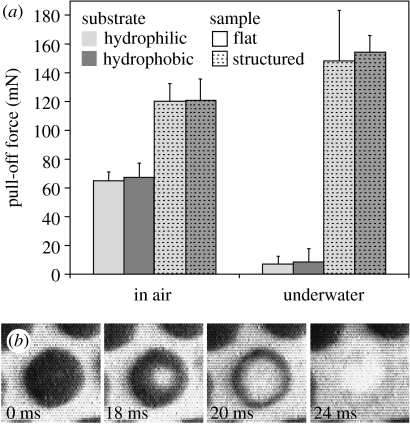
In contrast to the results obtained in air, where the structured surfaces revealed twice the effective pull-off force than the flat ones, the underwater adhesion of the structured surfaces was 20 times higher than that of the flat ones (figure 2a; table 1). Flat surfaces retained only 12% of their attachment ability in water when compared with their attachment ability in air. This reduction could not have been caused by changes in capillary attraction as similar attachment forces were measured on both hydrophilic and hydrophobic substrates (table 1), and is attributed to the effect of water on van der Waals dispersion forces. This is confirmed by the Hamaker constants (Israelachvili 1992) for the interaction of PVS and glass across air and water (Ha, 59×10−21 J, and Hw, 8×10−21 J, respectively), which predict an 86% decrease in van der Waals adhesion underwater, which closely matches the experimental results. Structured surfaces, however, gained in their attachment ability underwater, thus demonstrating some additional effect superimposed on van der Waals interaction.
Figure 2.
Adhesive performance of mushroom-shaped fibrillar microstructure. (a) Mean pull-off force of flat and structured samples measured on hydrophilic and hydrophobic substrates in air and de-ionized water. The error scales represent a standard deviation from mean values. Statistical significance of difference between the forces measured is summarized in table 1. (b) Behaviour of a single terminal contact plate detaching from substrate in air. Dark areas resulted from destructive interference of reflected white light in the glass–PVS interface visualize the real contact zones.
Table 1.
Statistical significance of difference between the pull-off forces presented in figure 2a.
| substrate | sample | environment | significant difference |
|---|---|---|---|
| hydrophobic versus hydrophilic | flat | air | no (p=0.727)a |
| hydrophobic versus hydrophilic | structured | air | no (p=0.941)a |
| hydrophobic versus hydrophilic | flat | water | no (p=1.000)b |
| hydrophobic versus hydrophilic | structured | water | no (p=1.000)b |
| both | flat versus structured | air | yes (p<0.001)a |
| both | flat versus structured | water | yes (p<0.001)a |
| both | flat | air versus water | yes (p<0.001)b |
| both | structured | air versus water | yes (p=0.007)a |
Calculated according to t-test (normal data distribution).
Calculated according to Mann–Whitney rank sum test (not normal data distribution).
As each mushroom-shaped microfibre is pulled off, a void is formed in the centre and grows towards the edge of the contact plate until it is finally detached from the substrate (figure 2b). Thus, despite being flat (figure 1 inset), each contact plate acts as a passive suction device when the thin, elastic contact plate lip does not allow the surrounding medium to enter the low-pressure zone formed due to the microfibre stalk tension. In water, which is sometimes described as having tensile strength, the decrease in pressure balances the expansive force until the pressure falls to the cavitation threshold (Smith 1991) and liquid water turns into vapour under the contact plate, thus allowing its deformation required for detachment. Estimating the effect of water on the pull-off force of the structured surface,
| (1.1) |
where Fa is the pull-off force in air (approximately 120 mN); pa and ps are the atmospheric and water saturation pressures (101 and 3 kPa, respectively) and Ac is the contact area of all microfibres (approx. 1.3 mm2), we have Fw=144 mN. In fact, water can sometimes withstand even negative pressures (Smith 1991) before cavitation occurs, so using the pressure of water saturation, ps, we might underestimate the effect of suction in water. From the other side, some microfibres can detach earlier, making the effective contact area, Ac, smaller, which might lead to the overestimation of the suction effect. Nevertheless, the estimated pull-off force is nearly identical to the mean measured pull-off force of 151 mN. This calculation not only explains a 25% increase in the attachment forces of our material when underwater but also sheds light on one of the functions of fluid secretion found on the mushroom-shaped fibrillar adhesive organs of some insects.
Interestingly, mushroom-shaped geometry of adhesive elements is also found in many marine organisms, such as mussels (Lin et al. 2007), sea stars (Flammang 1996), sea anemones (Bayer & Owre 1968), some algae (Mägdefrau 1992) and even bacteria (Tsang et al. 2006). Moreover, the most recent experiments reveal that mussels' adhesion, for instance, in some cases, is due to weak physical interactions rather than chemical bonding, and that the strong adhesion forces of mussels' attachment plaques arise as a consequence of their geometry rather than a high intrinsic surface or adhesion energy (Lin et al. 2007). This is consistent with the results presented here and might also be explained by a superposition of adhesive and cohesive effects.
4. Conclusion
The superposition of van der Waals dispersion force and suction effect affords new perspectives for surface micropatterning. We believe that the ability to generate glue-free reversible underwater adhesion through the use of contact splitting has practical potential in medicine, bio- and marine technologies and a range of applications in liquid-dominated environments.
Acknowledgments
We thank A.P. Summers for helpful comments and D. Voigt for providing silanized glass substrates. This work was supported by the Federal Ministry of Education, Science and Technology, Germany (project InspiRat 01RI0633C).
References
- Arzt E, Gorb S, Spolenak R. From micro to nano contacts in biological attachment devices. Proc. Natl Acad. Sci. USA. 2003;100:10 603–10 606. doi: 10.1073/pnas.1534701100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autumn K, Liang Y.A, Hsieh S.T, Zesch W, Chan W.P, Kenny T.W, Fearing R, Full R.J. Adhesive force of a single gecko foot-hair. Nature. 2000;405:681–685. doi: 10.1038/35015073. [DOI] [PubMed] [Google Scholar]
- Autumn K, et al. Evidence for van der Waals adhesion in gecko setae. Proc. Natl Acad. Sci. USA. 2002;99:12 252–12 256. doi: 10.1073/pnas.192252799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer F.M, Owre H.B. Macmillan; New York, NY: 1968. The free-living lower invertebrates. [Google Scholar]
- Beutel R.G, Gorb S.N. Ultrastructure of attachment specializations of hexapods (Arthropoda): evolutionary patterns inferred from a revised ordinal phylogeny. J. Zool. Syst. Evol. Res. 2001;39:177–207. doi: 10.1046/j.1439-0469.2001.00155.x. [DOI] [Google Scholar]
- Flammang P. Adhesion in echinoderms. In: Jangoux M, Lawrence J.M, editors. Echinoderm studies 5. A. A. Balkema; Rotterdam, The Netherlands: 1996. pp. 1–60. [Google Scholar]
- Geim A.K, Dubonos S.V, Grigorieva I.V, Novoselov K.S, Zhukov A.A, Shapoval S.Y. Microfabricated adhesive mimicking gecko foot-hair. Nat. Mater. 2003;2:461–463. doi: 10.1038/nmat917. [DOI] [PubMed] [Google Scholar]
- Gorb S.N. Springer; New York, NY: 2001. Attachment devices of insect cuticle. [Google Scholar]
- Gorb S, Varenberg M, Peressadko A, Tuma J. Biomimetic mushroom-shaped fibrillar adhesive microstructure. J. R. Soc. Interface. 2007;4:271–275. doi: 10.1098/rsif.2006.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelachvili J. Academic Press; New York, NY: 1992. Intermolecular and surface forces. pp. 176–212. [Google Scholar]
- Lin Q, Gourdon D, Sun C, Holten-Andersen N, Anderson T.H, Waite J.H, Israelachvili J.N. Adhesion mechanisms of the mussel foot proteins mfp-1 and mfp-3. Proc. Natl Acad. Sci. USA. 2007;104:3782–3786. doi: 10.1073/pnas.0607852104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mägdefrau K. Gustav Fischer; Stuttgart, Germany: 1992. Geschichte der Botanik. [Google Scholar]
- Smith A.M. Negative pressure generated by octopus suckers: a study of the tensile strength of water in nature. J. Exp. Biol. 1991;157:257–271. [Google Scholar]
- Smith A.M, Callow J.A, editors. Biological adhesives. Springer; Berlin, Germany: 2006. [Google Scholar]
- Stork N.E. Experimental analysis of adhesion of Chrysolina polita (Chrysomelidae: Coleoptera) on a variety of surfaces. J. Exp. Biol. 1980a;88:91–107. [Google Scholar]
- Stork N.E. Scanning electron microscope study of tarsal adhesive setae in the Coleoptera. Zool. J. Linn. Soc. Lond. 1980b;68:173–306. [Google Scholar]
- Tsang P.H, Li G, Brun Y.V, Freund L.B, Tang J.X. Adhesion in single bacterial cells in the micronewton range. Proc. Natl Acad. Sci. USA. 2006;103:5764–5768. doi: 10.1073/pnas.0601705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varenberg M, Gorb S. Shearing of fibrillar adhesive microstructure: friction and shear-related changes in pull-off force. J. R. Soc. Interface. 2007;4:721–725. doi: 10.1098/rsif.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite J.H. Nature's underwater adhesive specialist. Int. J. Adhes. Adhes. 1987;7:9–14. doi: 10.1016/0143-7496(87)90048-0. [DOI] [Google Scholar]