Abstract
Biodegradable polymer nanofibres have been extensively studied as cell culture scaffolds in tissue engineering. However, long-term in vitro studies of cell–nanofibre interactions were rarely reported and successful organ regeneration using tissue engineering techniques may take months (e.g. blood vessel tissue engineering). Understanding the long-term interaction between cells and nanofibrous scaffolds (NFS) is crucial in material selection, design and processing of the tissue engineering scaffolds. In this study, poly(l-lactide-co-ϵ-caprolactone) [P(LLA-CL)] (70 : 30) copolymer NFS were produced by electrospinning. Porcine coronary artery smooth muscle cells (PCASMCs) were seeded and cultured on the scaffold to evaluate cell–nanofibre interactions for up to 105 days. A favourable interaction between this scaffold and PCASMCs was demonstrated by cell viability assay, scanning electron microscopy, histological staining and extracellular matrix (ECM) secretion. Degradation behaviours of the scaffolds with or without PCASMC culture were determined by mechanical testing and gel permeation chromatography (GPC). The results showed that the PCASMCs attached and proliferated well on the P(LLA-CL) NFS. Large amount of ECM protein secretion was observed after 50 days of culture. Multilayers of aligned oriented PCASMCs were formed on the scaffold after two months of in vitro culture. In the degradation study, the PCASMCs were not shown to significantly increase the degradation rate of the scaffolds for up to 105 days of culture. The in vitro degradation time of the scaffold could be as long as eight months by extrapolating the results from GPC. These observations further supported the potential use of the P(LLA-CL) nanofibre in blood vessel tissue engineering.
Keywords: P(LLA-CL), coronary artery smooth muscle cell, electrospinning, nanofibres, long-term culture, extracellular matrix
1. Introduction
In the past decade there has been increased interest in electrospun nanofibrous structures for biomedical applications especially in tissue engineering (Reneker & Chun 1996; Deitzel et al. 2001). Fibres generated by electrospinning have diameters down to nanoscale dimensions, which are of the same order of magnitude as the physical dimensions of the nanoscaled protein fibrils in the natural extracellular matrix (ECM). In addition, this type of nanofibrous structure has high surface-to-volume ratio that potentially provides more anchoring points for cell attachment. These are properties that not only encourage cell growth and proliferation, but also promote tissue ingrowth and remodelling (Stevens & George 2005). Hence, extensive studies in recent years have evaluated the potentials of polymeric nanofibrous structures as tissue engineering scaffolds in biomedical applications (Matthews et al. 2002; Yoshimoto et al. 2003). A variety of polymers have been electrospun as the scaffolds and various applications have been proposed including skin, cartilage, bone and blood vessel tissue engineering (Boland et al. 2001; Huang et al. 2001; Stitzel et al. 2001; Li et al. 2002; Matthews et al. 2002; Yoshimoto et al. 2003).
In blood vessel tissue engineering, one approach to address this problem is to use a tissue-engineered artery graft, the main methods being listed in table 1. One method is to engineer a graft with a biodegradable scaffold. Niklason et al. (1999) first reported a functional tissue-engineered bovine artery, in which bovine vascular SMCs were seeded onto a biodegradable polyglycolic acid scaffold and cultured in dynamic bioreactor for eight weeks. The vessels were then seeded with endothelial cells (ECs) and further cultured prior to implantation. This tissue-engineered bovine arterial graft had a burst pressure exceeding 2000 mmHg. Recently, nanofibre has been used to construct vascular grafts (Hashi et al. 2007; Jeong et al. 2007). Jeong et al. seeded SMCs and ECs on collagen and poly(lactide-co-glycolide) (PLGA) nanofibre and cultured it for 23 days in a dynamic bioreactor. Hashi et al. (2007) reported a vascular graft by seeding SMCs and mesenchymal stem cells on poly(l-lactide) (PLLA) nanofibre followed by immediate implantation. All three approaches appear to lack the mechanical properties to justify long-term arterial implantation. One obvious strategy to increase strength is to simply increase the thickness or strength of the matrix. Despite the apparent simplicity of this approach, even the most advanced cell-seeded scaffold approaches have been unable to provide requisite strength to justify adult arterial implantation (Hibino et al. 2005). Niklason's work with long-term in vitro pre-conditioning, however, has shown some promise. Together, this suggests that a more in-depth understanding of the cell–matrix interactions during long-term in vitro culture may be important in blood vessel tissue engineering. Our laboratory has successfully electrospun nanofibrous scaffolds (NFS) from poly(l-lactide-co-ϵ-caprolactone) [P(LLA-CL)] copolymer and demonstrated their initial potential as scaffolds for vascular tissue engineering (Mo et al. 2004; Xu et al. 2004a,b). To further improve this approach in vascular tissue engineering, understanding of the long-term interaction between the cells and the biodegradable polymer P(LLA-CL) NFS is important in the design and processing of the NFS.
Table 1.
Current approaches to tissue-engineered vascular grafts.
| type | methods | best burst pressure | limitations | references |
|---|---|---|---|---|
| endothelialized synthetic grafts | seed ECs onto the intraluminal surface of a synthetic graft | depends on materials | non-biodegradable material would inhibit the vascular remodelling | Sipehia et al. (1996) and Deutsch et al. (1999) |
| collagen-based tissue-engineered vascular grafts | co-culture fibroblast, SMCs and ECs on collagen gels | <500 mmHg | the mechanical strength is below that required for artery (>2000 mmHg) | Weinberg & Bell (1986) and Huynh et al. (1999) |
| vascular graft grown in vivo | De novo create a tubular graft by inserting tubing into recipient's body | >2500 mmHg | no endothelium lining before transplantation. Risk of thrombosis | Campbell et al. (1999) and Chue et al. (2004) |
| cell-based tissue-engineered vascular grafts | SMCs and fibroblasts are cultured in monolayer, and then rolled to form tubular tissue structure, followed by EC seeding | >2000 mmHg | long culture time approximately three months | L'Heureux et al. (1998, 2004, 2006) |
| cell and scaffold-based tissue-engineered vascular grafts | vascular cells are seeded onto pre-shaped bio-compatible scaffold and cultured to form a vascular substitute | >2000 mmHg | complication from remaining polymer residue | Niklason et al. (1999, 2001), Buttafoco et al. (2006) and Heydarkhan-Hagvall et al. (2006) |
The degradation profile of the polymer is an important criterion to consider in choosing the appropriate material for successful applications in tissue engineering, especially for load-bearing structure in blood vessel applications. Ideally, a fully regenerated mechanical and biological competent tissue-engineered vascular graft requires complete scaffold degradation with well-defined cellular organization and tissue remodelling. However, biodegradable blocked polyesters were reported to induce inflammation and vigorous immunoresponse during degradation (Rihova 1996). Numerous studies have been conducted in recent years to evaluate the degradation behaviours of biodegradable polymer nanofibres. But most of these studies were conducted in vitro without cell culture, by immersing the nanofibres in buffered medium (Kim et al. 2003; Zong et al. 2003; Zeng et al. 2004; You et al. 2005), and a long-term degradation study of polymer nanofibres cultured with the cells in a controlled in vitro environment is not readily available. The effects of cell culture on the degradation behaviour of the polymer nanofibrous scaffold were also rarely studied.
In this study, biodegradable polymer P(LLA-CL) NFS were seeded with porcine coronary artery smooth muscle cells (PCASMCs) and cultured in vitro for up to 105 days, with the aim of evaluating the long-term viability of SMCs cultured on the scaffolds and to analyse the cellular effects on polymeric nanofibre degradation. We hypothesize that the degradation behaviour of polymeric polyester nanofibre will be milder than block materials, given the high surface area for diffusion of acidic degradation products. This will induce less inhibition on cell proliferation.
2. Material and methods
2.1 Materials and reagents
The block biodegradable copolymer of P(LLA-CL) (70 : 30, Mn=138 kDa, Resomer LC 703, Boehringer Ingelheim Pharma Chemicals, Germany), which has a composition of 70 mol% l-lactide and 30 mol% ϵ-caprolactone was used. Hexafluoroisopropanol (HFIP) of analytical grade was obtained from Sigma. PCASMCs were obtained at passage 2 from Cell Applications, Inc. (USA). All the cells used in the studies were under passage 6. All culture medium and reagents were purchased from Cell Applications, Inc. (USA).
2.2 Preparation of random P(LLA-CL) NFS by electrospinning
P(LLA-CL) solution of 12% (w/v) was prepared by dissolving P(LLA-CL) in HFIP (Sigma). The solution was placed in a plastic syringe fitted with a 27G one-half needle (Becton Dickinson, USA). An electrospinning voltage was applied between the needle and the collecting plate at 10 kV DC with a high voltage power supply (Gamma High Voltage Research, USA). The solution was delivered at a feed rate of 1.5 ml h−1. The resultant nanofibres were collected on an aluminium collector located at a distance of 15 cm from the needle tip. The collection time was 50 min and the average thickness of the nanofibre mesh was 44±10 (s.d.) μm. The thickness was determined by multi-point measurement using a micrometer (n>10). For degradation study with and without cell culture, the sample was cut into 4×8 cm2. For cell-viability test, the nanofibre was collected on glass coverslips (d=1.5 cm).
2.3 Culture of PCASMCs
The PCASMCs were cultured in PCASMC basal medium (Cell Applications, Inc., USA) supplemented with SMC growth supplements (Cell Applications, Inc., USA) and 10% foetal bovine serum. The medium was replaced every 2–4 days and the cultures were maintained in a humidified incubator at 37°C with 5% CO2. When the cells reached 80–90% confluence (5×104 cells cm−2), the cultures were trypsinized and sub-cultured at 1 : 3 ratios.
The P(LLA-CL) nanofibrous samples were sterilized in 70% ethanol solution for 30 min followed by three washes with 1× phosphate-buffered saline (PBS). The cells were seeded onto the NFS at a seeding density of 1.8×104 cells cm−2 and cultured for the desired duration to evaluate long-term cell–nanofibre interactions.
2.4 Scanning electron microscopy
Scanning electron microscopy (SEM) was used to examine the morphological details of the cells interacting with the P(LLA-CL) NFS following seeding for 5, 10, 15, 20, 30, 45, 70, 85 and 105 days. The cell–nanofibre samples were washed with 1× PBS to remove non-adherent cells. The adherent cells were fixed with 2.5% glutaraldehyde for 45 min at 4°C. Thereafter, the samples were sequentially dehydrated in 50, 70, 80, 90, 95 and 100% ethanol solutions for 10 min at each dehydration step and then dried under vacuum. The samples were sputter coated with gold (JEOL JFC-1200 Fine Coater, Japan) and observed using a field emission SEM (FEI Quanta 200, USA) at an accelerating voltage of 10 kV.
2.5 MTS assay for PCASMCs viability
For cell-viability test, the cells were seeded onto the NFS at a density of 1.8×104 cells cm−2 (30% confluence). After culturing the cells for 2, 5, 10, 18, 30 and 40 days, the unattached cells were washed out and the viability of the cells attached on the NFS was quantified by the colorimetric MTS assay (CellTiter 96 Aqueous One Solution Cell Proliferation Assay, Promega, USA). Briefly, the cell–nanofibre samples were incubated with 20% (v/v) 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium (MTS) reagent in complete medium for 4 hours. Thereafter, aliquots (in triplicates) were pipetted into a 96-well plate and reduction of the tetrazolium salt as indicated by colorimetric change was determined by measuring the absorbance at 490 nm with a spectrophotometric plate reader (FLUOstar OPTIMA, BMG Lab Technologies, Germany).
2.6 Bicinchoninic acid protein assay
The amount of ECM proteins secreted by the PCASMCs on NFS during long-term culture was measured using a bicinchoninic acid (BCA) protein assay kit (Pierce, USA; Stich 1990). Briefly, the PCASMCs were seeded onto the NFS at a density of 1.8×104 cells cm−2 (30% confluence). The culture medium was changed every 2–3 days. The PCASMCs cultured on P(LLA-CL) NFS for 15, 25, 40, 50, 70, 90 and 110 days were collected and trypsinized for 5 min and this was repeated three times to ensure complete removal of all the cells from the NFS as confirmed by SEM (data not shown). The NFS were then immersed in 0.1 ml PBS together with 2 ml BCA working reagent at room temperature for 1 hour, and the absorbance at 590 nm was measured with a spectrophotometric plate reader (FLUOstar OPTIMA, BMG Lab Technologies, Germany). The ECM protein concentration was determined from the bovine serum albumin standard curve.
2.7 In vitro degradation of the P(LLA-CL) NFS
Degradation of the P(LLA-CL) NFS was studied by immersing the scaffolds in pure culture medium or culturing with PCASMCs for various durations. At various intervals, the samples were collected and the mechanical properties of the wet samples were determined by tensile testing. To prepare dry samples for weigh loss, tensile testing and molecular weight determination, samples with or without the cells were trypsinized three times for 5 min each at 37°C and vacuum dried for 24 hours.
2.8 Mechanical property of the NFS
Tensile tests were performed using a 5848 microtester (Instron, Canton, MA, USA) at a stroke rate of 5 mm min−1 with a 20 mm gauge length on NFS cut in a rectangular (10×40 mm2) shape. The thicknesses were 44±10 μm. The thickness of each sample was determined by an average of three point measurement. This tensile test was performed in the same manner as standard mechanical tests for fabric materials (ASTM D4595).
2.9 Gel permeation chromatography
The number-average polymer molecular weights of the NFS were determined using gel permeation chromatography (GPC) equipped with a refractive index detector (Waters, Model 1515, Mildford, MA, USA). The P(LLA-CL) NFS cultured with PCASMCs for different durations were collected after three times of trypsinization (5 min) to completely remove the cells. The NFS were dissolved in tetrahydrofuran (THF; Sigma, USA), while the ECM components on the nanofibrous scaffold remained undissolved in the THF. GPC was performed at an elution flow rate of 1 ml min−1. Single Gaussian peaks were observed with no hints of ECM protein contaminations. Polystyrene standards (Polysciences, Warrington, PA, USA) were used to obtain a primary calibration curve.
3. Results
3.1 Morphology of the P(LLA-CL) nanofibre
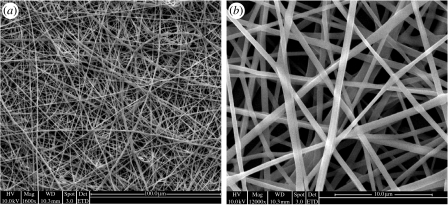
Using the biodegradable polymer P(LLA-CL), three-dimensional nanofibrous mesh was successfully prepared by electrospinning. Figure 1 shows the SEM micrographs of electrospun P(LLA-CL) NFS. The three-dimensional fibrous mesh comprised fibres with diameters ranging from 350 to 800 nm (average diameter=580 nm). The average thickness of the nanofibrous meshes was 44 μm.
Figure 1.
The SEM micrographs of P(LLA-CL) electrospun nanofibres with uniform fibre morphology. Magnification: (a) 1600× and (b) 12 000×.
3.2 Cell metabolism
3.2.1 Cell proliferation as determined by SEM and histology imaging
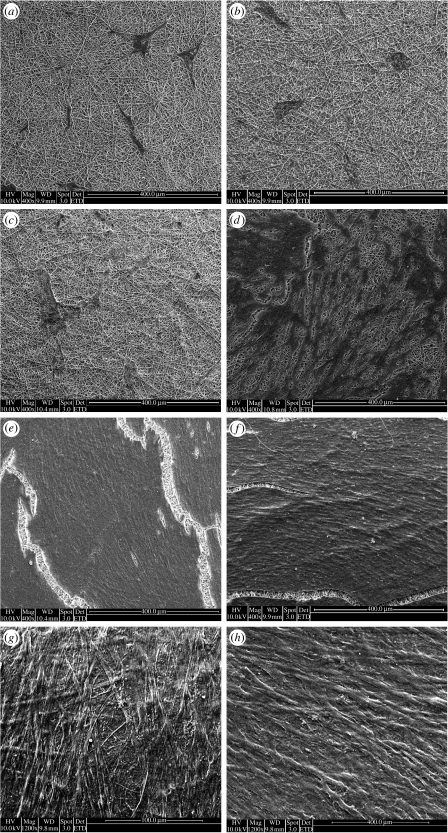
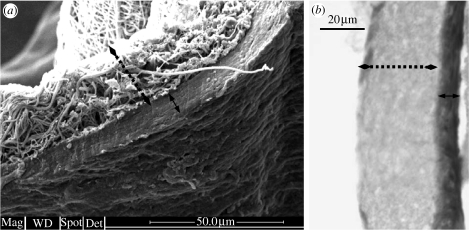
The growth of PCASMCs on the P(LLA-CL) NFS was initially slow but after approximately 15 days of culture an increase in proliferation rate was observed (figure 2). After two months of culture, the cells fully covered the P(LLA-CL) NFS in a randomly aligned manner (figure 2g). At 105 days of culture, the cells formed multiple cell layers on the scaffold, indicating a significant growth of the PCASMCs (figure 3). The SEM image shows that the PCASMC layer is approximately 10 μm in thickness.
Figure 2.
The PCASMCs were seeded at a density of 18 000 cells cm−2 on P(LLA-CL) NFS and cultured on for up to 105 days. The samples were harvested and imaged using SEM after various time intervals: (a) 5 days, (b) 10 days, (c) 17 days, (d) 30 days, (e) 45 days, (f) 70 days, (g) 85 days and (h) 105 days.
Figure 3.
After 105 days of culturing PCASMCs on the P(LLA-CL) NFS, the cell–scaffold construct was collected and subjected to (a) SEM and (b) histological staining. The solid two-way arrow indicates the cell layers and the broken arrow indicates the NFS. The SEM micrograph shows that the multiple cell layer that was approximately 10 μm in thickness formed an integral part of the scaffold.
3.2.2 MTS cell viability assay
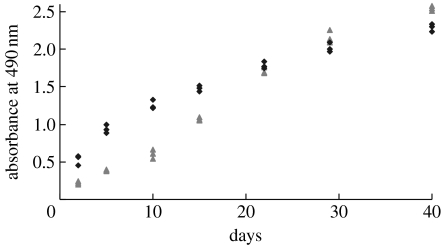
Figure 4 shows the cell viability assay of PCASMCs cultured on P(LLA-CL) NFS for up to 40 days. For short-term culture (less than 10 days), the P(LLA-CL) NFS were less conducive for initial cell growth when compared with tissue culture polystyrene surface (TCPS) (p<0.01). An increase in proliferation rate of PCASMCs cultured on P(LLA-CL) NFS was observed at approximately day 10 of culture. After approximately 30 days of culture, the viability of the cells cultured on P(LLA-CL) NFS was comparable to that of those cultured on TCPS. The increasing trend of cell viability was consistent with the observation from the SEM imaging.
Figure 4.
Viability of PCASMCs cultured on P(LLA-CL) NFS and TCPS for MTS assay. The PCASMCs were seeded at a density of 1.8×104 cells cm−2 and cultured for up to 40 days. Data were representative of three independent experiments and all data points were plotted (NFS, triangles; TCP, diamonds).
3.2.3 BCA assay for secreted ECM proteins
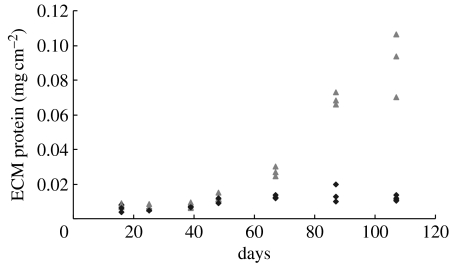
BCA assays were performed to quantify the amount of ECM proteins secreted by PCASMCs cultured on P(LLA-CL) NFS (figure 5). NFS immersed in pure culture medium served as controls to determine the absorption of proteins from the culture medium. Minimum amounts of soluble proteins from culture medium were adsorbed on to NFS as shown by the diamonds in figure 5. For the cells cultured on the P(LLA-CL) NFS, the amount of ECM proteins secreted by PCASMCs was not significant for cultures of less than 50 days. However, a significant increase in the amount of ECM protein secretion was observed starting from around day 50 of culture which increased sharply thereafter (p<0.01). The secreted ECM will benefit and contribute to the integrity of the cell–scaffold construct. The amount of ECM was increased 10 times from days 50 to 105. A thin layer of ECM was attached on the NFS after trypsinization, which was demonstrated by dissolving the nanofibre using THF (data not shown).
Figure 5.
BCA assay to quantify the ECM proteins secreted by PCASMCs seeded at 1.8×104 cells cm−2 on P(LLA-CL) NFS and cultured for up to 105 days. After various time intervals as depicted on the graph, the cell–scaffold constructs were collected, the cells were removed from the scaffolds by repeated trypsinization that was confirmed by SEM (data not shown). Then the scaffolds were assayed for the amount of ECM proteins secreted by the cells. The triangles show the ECM proteins secreted by the cells on the scaffolds. The scaffolds immersed in pure medium served as controls (diamonds). Data were representative of three independent experiments and all data points were plotted.
3.3 Material degradation
3.3.1 Mass loss of the scaffolds cultured with and without PCASMCs
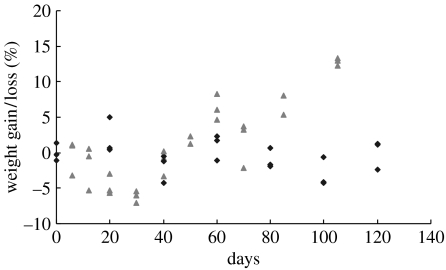
Mass loss of the P(LLA-CL) NFS was evaluated by immersing the scaffolds in culture medium for 120 days, and culturing PCASMCs for a period of 105 days. There was no significant mass loss of the P(LLA-CL) NFS immersed in the culture medium during the study period. In the first 30 days, there was a tendency that the scaffolds with cell culture lost some mass, but it was statistically insignificant (p>0.5). From 30 days onwards, increased weights were observed on the NFS with cell culture (figure 6). In summary, the P(LLA-CL) NFS cultured with PCASMCs showed an initial weight loss followed by a significant weight gain after 30 days of culture. The weight gain could be attributed to ECM accumulation on the scaffolds.
Figure 6.
Mass loss of P(LLA-CL) NFS cultured with PCASMCs (triangles) and immersed in culture medium (diamonds). Data were representative of three independent experiments and all data points were plotted.
3.3.2 Number-average molecular weight (Mn) of P(LLA-CL) during the degradation with/without PCASMC culture
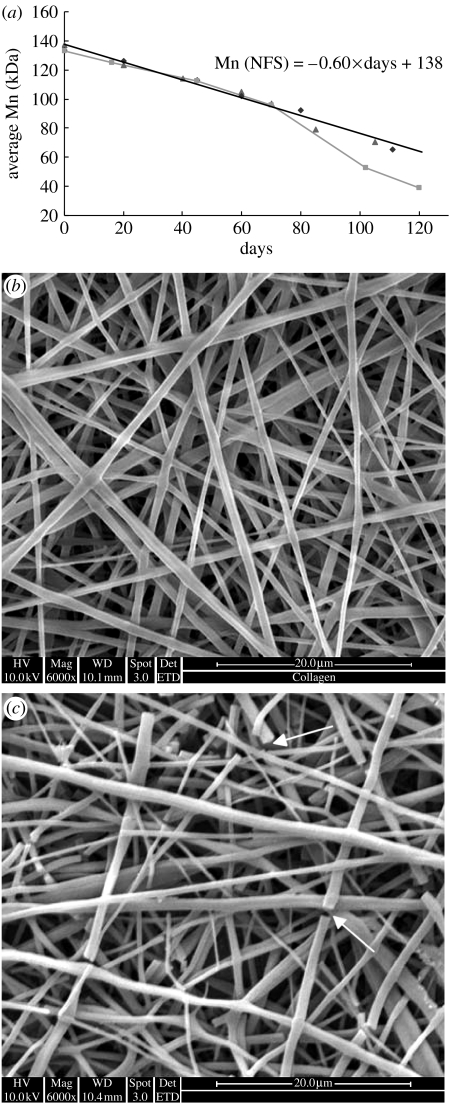
Figure 7a shows the molecular degradation profile of the P(LLA-CL) as determined by GPC. In contrast to a polymer block (squares; figure 7a) that exhibited an accelerated decrease in molecular weight after 60 days, the molecular weight of the P(LLA-CL) nanofibres decreased linearly during the degradation study. There was no obvious difference between the molecular weight loss of the scaffolds immersed in culture medium and cultured with PCASMCs, indicating that the cells may not have a significant influence on the degradation rate of bulk P(LLA-CL) nanofibres. Extrapolating the linear pattern of decrease in molecular weight of the polymer, it is estimated that the P(LLA-CL) nanofibres will completely degrade after approximately 230 days. This was substantiated by the SEM micrographs of the P(LLA-CL) nanofibres immersed in culture medium for 150 (figure 7b) and 210 days (figure 7c). After 210 days of degradation in the medium, the P(LLA-CL) nanofibres began to break and started to disintegrate.
Figure 7.
(a) Graph showing the number-average molecular weight (Mn) loss as determined by GPC for NFS cultured with PCASMCs (triangles), NFS immersed in culture medium (diamonds), and P(LLA-CL) pellet immersed in culture medium (squares). The Mn of P(LLA-CL) nanofibres decreased in a linear pattern as shown by the solid line. The SEM micrographs show the P(LLA-CL) nanofibres immersed in culture medium for (b) 150 days and (c) 210 days. The arrows show breakages in the nanofibres.
3.3.3 Mechanical characterization by tensile tests
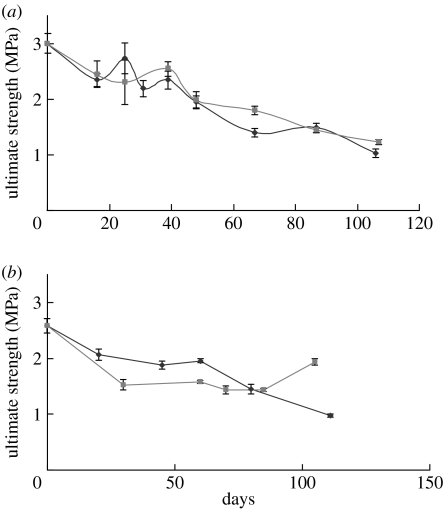
Tensile tests were performed on both the wet and dried scaffolds to determine the mechanical strength of the P(LLA-CL) NFS immersed in culture medium or cultured with PCASMCs. To prepare the dried scaffolds for tensile testing, the cells were removed from the scaffolds by trypsinization and the scaffolds were dried in vacuum for 24 hours at room temperature, while the wet scaffolds cultured with the cells were directly applied to tensile testing with the cells still attached to the scaffolds. For both the wet and dried scaffolds, a general decrease in the mechanical strength was observed when the P(LLA-CL) NFS were either immersed in culture medium or cultured with the cells (figure 8). In the dry test, the mechanical strength of scaffold cultured with PCASMCs decreased slightly faster than that of scaffold without cell culture in the first 80 days (figure 8b). However, an increase in the mechanical strength was observed for the scaffolds cultured with the cells for 105 days (figure 8b). But, such an increase was not observed in the wet test (figure 8a). The NFS cultured with PCASMCs showed similar mechanical strength drop compared with those immersed in culture medium. There is no significant difference in mechanical strength between the dry and wet tests.
Figure 8.
The ultimate strength of P(LLA-CL) NFS immersed in culture medium (diamonds) and cultured with PCASMCs (squares) as determined by tensile tests. Tensile tests were performed on cell-attached scaffolds under (a) wet condition and trypsinized scaffolds under (b) dry condition. Mean for n±s.d., n=3–6.
4. Discussion
NFS with topography that mimics the natural ECM structure have been demonstrated to enhance cell attachment, growth and proliferation (Xu et al. 2004a,b), and have been suggested as good substrates for tissue engineering applications in addition to the advantage of long-term structural integrity of polymer nanofibres depending on the choice of polymers. In this study, porcine coronary smooth muscle cells were demonstrated to have sustainable growth on the P(LLA-CL) NFS under long-term culture. Although initial cell viability on the P(LLA-CL) NFS was low, PCASMCs exhibited even higher cell viability than those cultured on TCPS after 30 days. Multiple cell layers were formed on the NFS after two months of culture. After three months of culture, 0.5 ml of culture medium was required to maintain the pH and multilayered cell viability per cm2 of culture area per day while a monolayer of 100% confluent cells cultured on the P(LLA-CL) NFS required only 0.1 ml of culture medium per cm2 of culture area per day (data not shown). The cells were observed to cohesively adhere to the scaffold as complete removal of the cells cultured on the NFS required three trypsinizations of 5 min each while the cells cultured on TCPS only required a single 2 min trypsinization.
An important factor in the success of tissue-engineered constructs is the ability of the cells to secrete ECM to replace and reinforce the structural integrity and strength of the constructs as the polymer is being degraded (Raines 2000; Ratcliffe 2000; Rosso et al. 2004). In blood vessel tissue engineering, mechanical properties such as tensile stiffness, elasticity, compressibility and viscoelasticity of the construct are important considerations for the success of the vascular graft, and the ECM contents such as collagens, elastin and proteoglycans secreted by the cells in graft maturation contribute to these physical and mechanical properties of the vascular grafts (Raines 2000; Ratcliffe 2000). Coronary artery smooth muscle cells have been shown to possess the ability to secrete their own ECM components (Davies & Hagen 1994; Allaire & Clowes 1997; Newby & Zaltsman 2000; Fitzsimmons & Shanahan 2002; MacNeill et al. 2002). It is essential that these cells are able to produce and secrete ECM components to replace the polymer scaffold as it degrades during graft maturation. In this study, total ECM proteins secreted by PCASMCs on the NFS were evaluated. The amount of ECM deposited on the scaffolds increased dramatically from day 50 of the culture. After three months of culturing PCASMCs on the NFS, the amount of ECM deposition on the scaffold was more than 10-fold higher compared with day 50 of culture. Even after prolonged treatment with trypsin for complete cell removal from the scaffolds after three months of culture, the remaining amount of ECM proteins bound to the scaffolds was as high as 0.1±10% mg cm−2. This could be explained by the high secretion of ECM after 50 days of culture. It is also possible that the ECM tends to resist trypsinization by cross-linking after 50 days of culture. The actual amount of ECM secreted by PCASMCs (before trypsinization) could be much higher than that. The high level of ECM could also explain the increased mechanical strength (dried condition) of the scaffold after three months of cell culture. However, the mechanical strength of the cell–scaffold constructs remained poor in wet conditions, and this may be explained by the random orientation of the PCASMCs on the scaffolds and the elastic nature of the ECM components in wet conditions. The consolidated ECM increased the strength of scaffold in dry conditions.
It was also observed that PCASMCs cultured on the scaffolds did not significantly increase the degradation rate of the scaffolds, and that the NFS still supported cell growth while degrading. The molecular weight of the NFS either immersed in culture medium or cultured with the cells decreased linearly with very similar rates for the duration of the study. In contrast, in the dry mechanical test, the scaffolds cultured with the cells showed a faster decrease in the mechanical strength compared with those immersed in culture medium for the first 80 days. But in the wet test, the scaffolds with and without the cells showed an equal decrease in mechanical strength throughout the study period. If the scaffolds with the cells lost strength faster than those without cells as shown by dry mechanical test, it is suspected that the cells and ECM components on the scaffold contributed to the overall mechanical strength of the cell–nanofibrous scaffold construct in the wet test. Therefore, we suspected that there might be mild surface erosion on the scaffolds induced by the adherent PCASMCs, leaving the bulk volume of the NFS unaffected. The fact that the NFS cultured with PCASMCs showed faster mass loss until day 30 (though mass increase was observed later due to ECM secretion) further supported our postulation of surface erosion. Taken together, although the P(LLA-CL) NFS cultured with or without PCASMCs showed similar trends of decrease in Mn, PCASMCs could increase the polymer degradation by surface erosion.
Although the polymer pellets showed a similar rate of molecular weight loss as compared to NFS during the first 60 days of the degradation study, a sharp decrease in the molecular weight of the polymer pellets was observed thereafter, while the NFS still degraded linearly. One tentative explanation is the build-up of the acidic microenvironment within the polymer blocks which accelerates degradation by autocatalysis. This was not observed in NFS as the acidic degradation products could easily diffuse out of the nanofibres (Shin et al. 2006). It is further postulated that the linear degradation profile of the NFS might indicate that the major pattern of molecular degradation is unzipping of the molecule chains (i.e. degradation from the ends of polymer chains), rather than random scission, which usually happens in autocatalysis (Hakkarainen 2002). The build-up of acidic microenvironment in biodegradable polymer scaffolds has been shown to increase the rate of cell mitosis (Higgins et al. 2003) or induce foreign body immune response (van der Elst et al. 1999; You et al. 2005). Hence, the linear degradation profile of the P(LLA-CL) NFS with minimal build-up of acidic microenvironment is well suited for culturing coronary artery smooth muscle cells.
Although PCASMCs were cultured on P(LLA-CL) NFS for 105 days in this study, the cell–scaffold constructs did not provide satisfactory mechanical strength and structural integrity. There is also concern that the tensile testing results cannot be directly translated to burst pressure of vascular graft. The culture time was long and the cell–scaffold constructs lacked sufficient mechanical strength. However, after two months of culturing PCASMCs on 2.5×7.5 cm2 P(LLA-CL) NFS, the cell number increased almost 20-fold from 3×105 to approximately 6×106 cells (data not shown), which was comparable to the tissue-engineered vascular graft developed in vitro in a dynamic bioreactor by Niklason et al. (1999). An earlier study in our laboratory has shown that human coronary artery smooth muscle cells aligned along the orientation of the nanofibres when cultured on aligned NFS (Xu et al. 2004a). In native blood vessels, the smooth muscle cells are organized in a concentric manner to provide mechanical strength and structural integrity to the vessels. Hence, one approach to engineer a vascular graft is to culture SMCs on aligned NFS to mimic the circumferential organization of SMCs in native arteries, and the cell–scaffold construct could be rolled into tubular structure and developed in a dynamic bioreactor. This would potentially shorten the culturing time of the vascular graft and impart the desired mechanical strength and structural integrity to the tissue-engineered vascular graft.
5. Conclusion
In this study, we cultured PCASMCs on P(LLA-CL) NFS for up to 105 days and characterized cell viability, ECM protein secretion and the degradation profile of the NFS. Our results demonstrated that (i) nanofibrous P(LLA-CL) scaffolds provided a favourable environment for PCASMC long-term proliferation, (ii) the NFS supported multilayered cell growth with a significant amount of ECM protein secretion, (iii) cell growth did not significantly increase the degradation rate of the polymer nanofibres and (iv) the P(LLA-CL) nanofibre exhibited a linear and slower molecular weight loss when compared with polymer block materials.
Acknowledgments
This study was supported by the Ministry of Education, Singapore, and Office of Life Science, National University of Singapore.
References
- Allaire E, Clowes A.W. Endothelial cell injury in cardiovascular surgery: the intimal hyperplastic response. Ann. Thorac. Surg. 1997;63:582–591. doi: 10.1016/S0003-4975(96)01045-4. [DOI] [PubMed] [Google Scholar]
- Boland E.D, Wnek G.E, Simpson D.G, Pawlowski K.J, Bowlin G.L. Tailoring tissue engineering scaffolds using electrostatic processing techniques: a study of poly(glycolic acid) electrospinning. J. Macromol. Sci. Pure Appl. Chem. 2001;38:1231–1243. [Google Scholar]
- Buttafoco L, Engbers-Buijtenhuijs P, Poot A.A, Dijkstra P.J, Vermes I, Feijen J. Physical characterization of vascular grafts cultured in a bioreactor. Biomaterials. 2006;27:2380–2389. doi: 10.1016/j.biomaterials.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Campbell J.H, Efendy J.L, Campbell G.R. Novel vascular graft grown within recipient's own peritoneal cavity. Circ. Res. 1999;85:1173–1178. doi: 10.1161/01.res.85.12.1173. [DOI] [PubMed] [Google Scholar]
- Chue W.L, Campbell G.R, Caplice N, Muhammed A, Berry C.L, Thomas A.C, Bennett M.B, Campbell J.H. Dog peritoneal and pleural cavities as bioreactors to grow autologous vascular grafts. J. Vasc. Surg. 2004;39:859–867. doi: 10.1016/j.jvs.2003.03.003. [DOI] [PubMed] [Google Scholar]
- Davies M.G, Hagen P.O. Pathobiology of intimal hyperplasia. Br. J. Surg. 1994;81:1254–1269. doi: 10.1002/bjs.1800810904. [DOI] [PubMed] [Google Scholar]
- Deitzel J.M, Kleinmeyer J, Harris D, Tan N.C.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer. 2001;42:261–272. doi: 10.1016/S0032-3861(00)00250-0. [DOI] [Google Scholar]
- Deutsch M, Meinhart J, Fischlein T, Preiss P, Zilla P. Clinical autologous in vitro endothelialization of infrainguinal ePTFE grafts in 100 patients: a 9-year experience. Surgery. 1999;126:847–855. [PubMed] [Google Scholar]
- Fitzsimmons C.M, Shanahan C.M. Vascular extracellular matrix. In: Lanzer P, Topol E.J, editors. Pan vascular medicine: integrated clinical management. Springer; Berlin, Germany; New York, NY: 2002. pp. 217–231. [Google Scholar]
- Hakkarainen M. Aliphatic polyesters: abiotic and biotic degradation and degradation products. Adv. Polym. Sci. 2002;157:113–138. [Google Scholar]
- Hashi C.K, Zhu Y.Q, Yang G.Y, Young W.L, Hsiao B.S, Wang K, Chu B, Li S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc. Natl Acad. Sci. USA. 2007;104:11 915–11 920. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydarkhan-Hagvall S, Esguerra M, Helenius G, Soderberg R, Johansson B.R, Risberg B. Production of extracellular matrix components in tissue-engineered blood vessels. Tissue Eng. 2006;12:831–842. doi: 10.1089/ten.2006.12.831. [DOI] [PubMed] [Google Scholar]
- Hibino N, Shin'oka T, Matsumura G, Ikada Y, Kurosawa H. The tissue-engineered vascular graft using bone marrow without culture. J. Thorac. Cardiovasc. Surg. 2005;129:1064–1070. doi: 10.1016/j.jtcvs.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Higgins S.P, Solan A.K, Niklason L.E. Effects of polyglycolic acid on porcine smooth muscle cell growth and differentiation. J. Biomed. Mater. Res. Part A. 2003;67A:295–302. doi: 10.1002/jbm.a.10599. [DOI] [PubMed] [Google Scholar]
- Huang L, Apkarian R.P, Chaikof E.L. High-resolution analysis of engineered type I collagen nanofibers by electron microscopy. Scanning. 2001;23:372–375. doi: 10.1002/sca.4950230603. [DOI] [PubMed] [Google Scholar]
- Huynh T, Abraham G, Murray J, Brockbank K, Hagen P.O, Sullivan S. Remodeling of an acellular collagen graft into a physiologically responsive neovessel. Nat. Biotechnol. 1999;17:1083–1086. doi: 10.1038/15062. [DOI] [PubMed] [Google Scholar]
- Jeong S.I, Kim S.Y, Cho S.K, Chong M.S, Kim K.S, Kim H, Lee S.B, Lee Y.M. Tissue-engineered vascular grafts composed of marine collagen and PLGA fibers using pulsatile perfusion bioreactors. Biomaterials. 2007;28:1115–1122. doi: 10.1016/j.biomaterials.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Kim K, Yu M, Zong X.H, Chiu J, Fang D.F, Seo Y.S, Hsiao B.S, Chu B, Hadjiargyrou M. Control of degradation rate and hydrophilicity in electrospun non-woven poly(d,l-lactide) nanofiber scaffolds for biomedical applications. Biomaterials. 2003;24:4977–4985. doi: 10.1016/S0142-9612(03)00407-1. [DOI] [PubMed] [Google Scholar]
- L'Heureux N, Paquet S, Labbe R, Germain L, Auger F.A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- L'Heureux N, et al. First use of a completely biological human tissue engineered blood vessel in a primate model. Circulation. 2004;110:508. doi: 10.1161/01.CIR.0000136821.99814.43. [DOI] [PubMed] [Google Scholar]
- L'Heureux N, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat. Med. 2006;12:361–365. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.J, Laurencin C.T, Caterson E.J, Tuan R.S, Ko F.K. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- MacNeill B.D, Pomerantseva I, Lowe H.C, Oesterle S.N, Vacanti J.P. Toward a new blood vessel. Vasc. Med. 2002;7:241–246. doi: 10.1191/1358863x02vm433ra. [DOI] [PubMed] [Google Scholar]
- Matthews J.A, Wnek G.E, Simpson D.G, Bowlin G.L. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- Mo X.M, Xu C.Y, Kotaki M, Ramakrishna S. Electrospun P(LLA-CL) nanofiber: a biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials. 2004;25:1883–1890. doi: 10.1016/j.biomaterials.2003.08.042. [DOI] [PubMed] [Google Scholar]
- Newby A.C, Zaltsman A.B. Molecular mechanisms in intimal hyperplasia. J. Pathol. 2000;190:300–309. doi: 10.1002/(SICI)1096-9896(200002)190:3%3C300::AID-PATH596%3E3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Niklason L.E, Gao J, Abbott W.M, Hirschi K.K, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- Niklason L.E, Abbott W, Gao J.M, Klagges B, Hirschi K.K, Ulubayram K, Conroy N, Jones R. Morphologic and mechanical characteristics of engineered bovine arteries. J. Vasc. Surg. 2001;33:628–638. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- Raines E.W. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int. J. Exp. Pathol. 2000;81:173–182. doi: 10.1046/j.1365-2613.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe A. Tissue engineering of vascular grafts. Matrix Biol. 2000;19:353–357. doi: 10.1016/S0945-053X(00)00080-9. [DOI] [PubMed] [Google Scholar]
- Reneker D.H, Chun I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology. 1996;7:216–223. doi: 10.1088/0957-4484/7/3/009. [DOI] [Google Scholar]
- Rihova B. Biocompatibility of biomaterials: hemocompatibility, immunocompatibility and biocompatibility of solid polymeric materials and soluble targetable polymeric carriers. Adv. Drug Deliv. Rev. 1996;21:157–176. doi: 10.1016/S0169-409X(96)00404-8. [DOI] [Google Scholar]
- Rosso F, Giordano A, Barbarisi M, Barbarisi A. From cell–ECM interactions to tissue engineering. J. Cell. Physiol. 2004;199:174–180. doi: 10.1002/jcp.10471. [DOI] [PubMed] [Google Scholar]
- Shin H.J, Lee C.H, Cho I.H, Kim Y.J, Lee Y.J, Kim I.A, Park K.D, Yui N, Shin J.W. Electrospun PLGA nanofiber scaffolds for articular cartilage reconstruction: mechanical stability, degradation and cellular responses under mechanical stimulation in vitro. J. Biomater. Sci. Polym. Ed. 2006;17:103–119. doi: 10.1163/156856206774879126. [DOI] [PubMed] [Google Scholar]
- Sipehia R, Martucci G, Lipscombe J. Transplantation of human endothelial cell monolayer on artificial vascular prosthesis: the effect of growth-support surface chemistry, cell seeding density, ECM protein coating, and growth factors. Artif. Cells Blood Substit. Immobil. Biotechnol. 1996;24:51–63. doi: 10.3109/10731199609117431. [DOI] [PubMed] [Google Scholar]
- Stevens M.M, George J.H. Exploring and engineering the cell surface interface. Science. 2005;310:1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- Stich T.M. Determination of protein covalently bound to agarose supports using bicinchoninic acid. Anal. Biochem. 1990;191:343–346. doi: 10.1016/0003-2697(90)90229-3. [DOI] [PubMed] [Google Scholar]
- Stitzel J.D, Pawlowski K.J, Wnek G.E, Simpson D.G, Bowlin G.L. Arterial smooth muscle cell proliferation on a novel biomimicking, biodegradable vascular graft scaffold. J. Biomater. Appl. 2001;16:22–33. doi: 10.1106/U2UU-M9QH-Y0BB-5GYL. [DOI] [PubMed] [Google Scholar]
- van der Elst M, Klein C.P.A.T, de Blieck-Hogervorst J.M, Patka P, Haarman H.J.T.M. Bone tissue response to biodegradable polymers used for intramedullary fracture fixation: a long-term in vivo study in sheep femora. Biomaterials. 1999;20:121–128. doi: 10.1016/S0142-9612(98)00117-3. [DOI] [PubMed] [Google Scholar]
- Weinberg C.B, Bell E. A blood-vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- Xu C.Y, Inai R, Kotaki M, Ramakrishna S. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials. 2004a;25:877–886. doi: 10.1016/S0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- Xu C.Y, Inai R, Kotaki M, Ramakrishna S. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 2004b;10:1160–1168. doi: 10.1089/ten.2004.10.1160. [DOI] [PubMed] [Google Scholar]
- Yoshimoto H, Shin Y.M, Terai H, Vacanti J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–2082. doi: 10.1016/S0142-9612(02)00635-X. [DOI] [PubMed] [Google Scholar]
- You Y, Min B.M, Lee S.J, Lee T.S, Park W.H. In vitro degradation behavior of electrospun polyglycolide, polylactide, and poly(lactide-co-glycolide) J. Appl. Polym. Sci. 2005;95:193–200. doi: 10.1002/app.21116. [DOI] [Google Scholar]
- Zeng J, Chen X.S, Liang Q.Z, Xu X.L, Jing X.B. Enzymatic degradation of poly(l-lactide) and poly(epsilon-caprolactone) electrospun fibers. Macromol. Biosci. 2004;4:1118–1125. doi: 10.1002/mabi.200400092. [DOI] [PubMed] [Google Scholar]
- Zong X.H, Ran S.F, Kim K.S, Fang D.F, Hsiao B.S, Chu B. Structure and morphology changes during in vitro degradation of electrospun poly(glycolide-co-lactide) nanofiber membrane. Biomacromolecules. 2003;4:416–423. doi: 10.1021/bm025717o. [DOI] [PubMed] [Google Scholar]