Abstract
Background
Platelets are thought to play a role in a variety of inflammatory conditions in the lung, some of which may lead to fibrosis. In the current study we tested the hypothesis that whole platelets and platelet lysate can mediate remodelling of extracellular matrix in vitro by affecting fibroblast-mediated contraction of a collagen gel. We also sought to determine to what extent platelet-derived growth factor (PDGF) and transforming growth factor-β (TGF-β) contribute to this effect.
Methods
Washed platelets, isolated from healthy blood donors, and platelet lysate (freezing and thawing), were cast together with human lung fibroblasts in three-dimensional collagen gels. The gels were then released and cultured for four days. PDGF and TGF-β1 concentrations were measured in culture supernatants by ELISA.
Results
Both platelets and platelet lysate augmented fibroblast-mediated gel contraction in a time and concentration dependent manner (19.9% ± 0.1 (mean ± SEM) of initial area vs. 48.0% ± 0.4 at 48 hours; P < 0.001 and 41.5% ± 0.6 vs. 60.6% ± 0.3 at 48 hours; P < 0.001, respectively). Fixed platelets had no effect in the system. Both TGF-β1 and PDGF-AA/AB were released in co-culture. PDGF-AA/AB had a maximum release at 24 hours whereas TGF-β1 release increased with longer culture periods. Neutralising antibodies to these mediators partially inhibited platelet-induced gel contraction.
Conclusion
We conclude that platelets may promote remodelling of extracellular matrix in vitro and that PDGF and TGF-β partially mediate this effect, also indicating a role for other mediators. The findings may be an important mechanism in regulating repair processes after injury.
Keywords: platelets, gel contraction, fibrosis, PDGF, TGF-β
Introduction
Platelets have a major function in maintaining homeostasis by initiating the coagulation process. In addition, activated platelets have a capacity to participate in complex cellular interactions. For instance, platelets constitute and release a variety of mediators that can modulate endothelial permeability and recruit inflammatory cells [1]. This local inflammatory process also enables circulating platelets to enter the extra vascular milieu and to adhere to exposed matrix via integrins that bind to collagen and laminin [2].
Fibroblasts are the major type of mesenchymal cells present in the connective tissue matrix [3]. Besides being a structural cell, the fibroblast can secrete a number of inflammatory mediators, which have the potential to drive fibrotic tissue remodelling. This is a complex process consisting of recruitment and proliferation of fibroblasts and production of extracellular matrix components. Part of this process includes contraction of matrix and can be present not only in scar formation but also in most fibrotic conditions [4,5]. Culturing of fibroblasts in three-dimensional native type I collagen gels has been used to model this contractile process and is considered to be one aspect of fibrotic tissue remodelling [6,7].
In certain conditions, platelets may accumulate as a part of the inflammatory response. For instance, in acute respiratory distress syndrome (ARDS) platelets are initially sequestered in the pulmonary microvasculature where they release substances that promote vaso- and broncho-constriction [8]. Histologically ARDS is characterised by an intense inflammation in the lung, which may progress to pulmonary fibrosis.
Platelet-derived growth factor (PDGF) and transforming growth factor-β (TGF-β) constitute two potential platelet-associated mediators promoting fibrosis. Previous studies have shown that both these mediators can stimulate fibroblast-mediated contraction of collagen gels [4,9,10]. In the current study we tested the hypothesis that whole platelets and platelet lysate could augment contraction of collagen gels in vitro. We also wanted to evaluate the relative contribution of PDGF and TGF-β in mediating this effect.
Materials and methods
Materials
Type I collagen (rat-tail tendon collagen, RTTC) was extracted according to a previously published method [11]. Briefly, tendons were excised from rat-tails, and the tendon sheath and other connective tissues were carefully removed. After repeated washing with Tris-buffered saline (0.9% NaCl, 10 mM Tris, pH 7.5) the tendons were washed in increasing concentrations of ethanol. Type I collagen was then extracted in 6 mM acetic acid. Protein concentration was determined by weighing a lyophilised aliquot from each lot of collagen. The RTTC was stored at 4°C until use.
Cell culture
Human foetal lung fibroblasts (HFL1) were obtained from the American Type Culture Collection (Rockville, MD, USA). The cells were cultured on 100-mm tissue culture dishes (FALCON; Franklin Lakes, NJ, USA) with Dulbecco's Modified Eagle Medium (D-MEM; GIBCOBRL/Lifetechnologies, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS; GIBCOBRL/Lifetechnologies), 50 U/mL penicillin G sodium, 50 μg/mL streptomycin sulphate (penicillin-streptomycin, GIBCOBRL/Lifetechnologies) and 1 μg/mL amphotericin B (fungizone, GIBCOBRL/Lifetechnologies). The fibroblasts were passaged twice a week and were used between the 15th and 20th passage. Confluent fibroblasts were trypsinised (Trypsin-EDTA; GIBCOBRL/Lifetechnologies, 0.05% trypsin 0.53 mM EDTA), resuspended in D-MEM without serum and used for collagen gel culture.
Platelets
Platelets were obtained from healthy blood donors by an apheresis procedure, using a blood cell separator (Cobe BCT Inc, Lakewood, CO, USA) and a closed-system apheresis kit (extended life platelet dual needle set, Cobe). This system generates a leukocyte-depleted concentrate (< 1 × 106 leukocytes/L). The platelet concentrates were stored in a computerised environmental chamber (Melco Engineering, CA, USA) at 21.6–22.5°C with constant linear agitation, 60 rpm. Samples were drawn under sterile conditions from each bag. The platelets were centrifuged at 900 × g for 15 minutes and then washed four times (850 × g, 10 min) in 5 mL sterile phosphate-buffered saline (PBS) to get rid of plasma. Finally, the platelets were resuspended in PBS and counted in a Micro Diff II Hematology analyzer (Beckman Coulter; Fullerton, CA, USA).
Fixation and lysing of platelets
Washed platelets were resuspended in 4% paraformaldehyde (PFA), incubated for 10 minutes in room temperature and washed three times (900 × g, 5 min) in PBS. The fixed cells were resuspended in PBS to various concentrations. To obtain platelet lysate the platelets were lysed by freezing (-20°C) and thawing three times. The cell debris was removed by centrifugation and the platelet lysate (supernatant) was stored in -20°C until use. The lysate contained significant concentrations of PDGF and TGF-β (approximately 500 ng/mL lysate for both, measured by ELISA, see below).
Collagen gel contraction assay
Collagen gels were prepared as previously described [12]. Briefly, distilled water, RTTC, 4 × D-MEM, fibroblast- and platelet-suspensions were mixed together to a final concentration of 0.75 mg/mL of collagen, 3 × 105 fibroblasts/mL and physiologic ionic strength of 1 × D-MEM. Platelets were added in various concentrations from 1 × 106–1 × 108 platelets/mL gel. The different mixtures were kept on ice during the preparation. Five hundred and fifty μL of the gel solution were then cast into each well of a 24-well tissue culture plate with a 2-cm2 growth area (FALCON). Gelation occurred within 30 minutes at 37°C.
The ability of co-cultured platelets to affect fibroblast-mediated collagen gel contraction was determined by the slow contraction assay of Bell et al. [6]. Fibroblasts and platelets in co-culture were prepared as described above. After gelation the gels were released from the surface of the culture well using a sterile spatula and transferred into 60-mm tissue culture dishes (FALCON) containing 5 mL of serum-free D-MEM. The floating gels were incubated at 37°C and 5% CO2 for four days and the gel area were measured daily. The same preparation procedure was performed when fixed platelets or platelet lysate were tested in the system.
Measurement of gel area
In all the experiments, the area of the collagen gels was measured by an image analyser system (LEICA Microsystems AG; Wetzlar, Germany). The images were recorded by a video camera comprising a zoom lens mounted approximately 15 cm above a lighted stage. Sixty-mm culture dishes rested on this stage, and the gels were observed directly. No special mounting was required for stability. The area of the gels was then captured and processed by computer software from LEICA Microsystems AG and collagen gel contraction in the horizontal axes was determined. Thickness was not measured and contraction in the vertical area was not assessed. With the described method, the area of each gel could be measured while sterility was preserved.
DNA content
In order to estimate cell numbers in the gels, the DNA content was assayed fluorometrically with Hoechst dye 33258 (SIGMA-ALDRICH, St Louis, Missouri, USA) by a modification of a previously published method [13]. Briefly, the gels were dissolved in 0.5 mL collagenase (GIBCOBRL/Lifetechnologies, 0.25 mg/mL) by incubation at 37°C for 2–3 h [14]. Cells were then collected by centrifugation (1500 × g, 10 min). After freezing and thawing once, 1 mL of ddH2O was added to each sample and cells were sonicated. The DNA content was determined by adding 2 mL of TNE buffer (10 mM Tris, 3 M NaCl, and 1.5 mM EDTA, pH 7.4) containing 2 μg/mL of Hoechst dye 33258. Fluorescence intensities were measured with a fluorescence spectrometer (Fluroscan 2; Labsystems, Finland) with an excitation wavelength at 355 nm and an emission wavelength at 460 nm.
Determination of PDGF-AA/AB and TGF-β1 in culture medium
Floating gels containing fibroblasts and co-culture of fibroblasts and viable, fixed or lysed platelets were cultured in 5 mL serum free D-MEM in 60-mm tissue dishes (FALCON). After two days the media surrounding the gels were collected and the samples were analysed for PDGF-AB and TGF-β1 by commercially available quantitative sandwich enzyme immunoassay, ELISA (PDGF-AB; Quantikine®, Human PDGF-AB Immunoassay, TGF-β1; Quantikine®, Human TGF-β1 Immunoassay, R&D Systems, Minneapolis, MN, USA).
In additional time-course experiments PDGF-AB, PDGF-AA (PDGF-AA; Quantikine®, Human PDGF-AB Immunoassay, R&D Systems) and TGF-β1 were determined in the surrounding media from co-culture of fibroblast and whole platelets after 2, 12, 24 and 48 hours of culture by ELISA. The TGF-β1 ELISA was performed as previously described by Kim et al. [15]. Briefly, microtiter plates (VWR International, Stockholm, Sweden) were coated with monoclonal anti-human TGF-β1 antibodies (clone 9016, R&D Systems). Standard and samples were added and incubated followed by addition of biotinylated anti-TGF-β1 antibodies (200 ng/mL, R&D Systems). Then HRP-streptavidin (1:200, R&D Systems) was applied. Finally the substrate TMB (3,3',5,5'-tetra methylbenzidine, SIGMA-ALDRICH) was added and allowed to develop. TGF-β1 was measured with or without activation by acidification in both assays. The optical density (OD) was determined by a spectrophotometer (Anthos 2001, Labdesign, Täby, Sweden) by measuring absorbance at 450 nm.
Neutralisation of human PDGF and TGF-β bioactivity
Neutralising antibodies to PDGF and TGF-β were used to evaluate the relative contribution of these two agents to fibroblast-mediated collagen gel contraction induced by platelets. Anti-human PDGF antibody, pan-specific anti-TGF-β polyclonal antibody (R&D Systems, Europe, Ltd., Abingdon, Oxon, UK) were pre-incubated with platelets 30 minutes in room temperature before mixed in the gels. Antibodies were also added to the media surrounding the gels (final concentration 25 μg anti-PDGF/mL and 50 μg anti-TGF-β/mL). An irrelevant rabbit IgG antibody (R&D Systems) was also tested (final concentration 50 μg/mL). Gels were cultured for two days and gel area was measured as described in Measurement of gel area.
To check the efficiency of the blocking antibodies in the gels PDGF and TGF-β were added to collagen gels and surrounding medium. A concentration of PDGF and TGF-β equal to that assayed in fibroblast/platelet co-culture (see Figure 7, HFL1/Plt) augmented fibroblast-mediated collagen gel contraction by approximately five percent. This augmentation could be neutralised by the addition of antibodies to PDGF and TGF-β.
Figure 7.
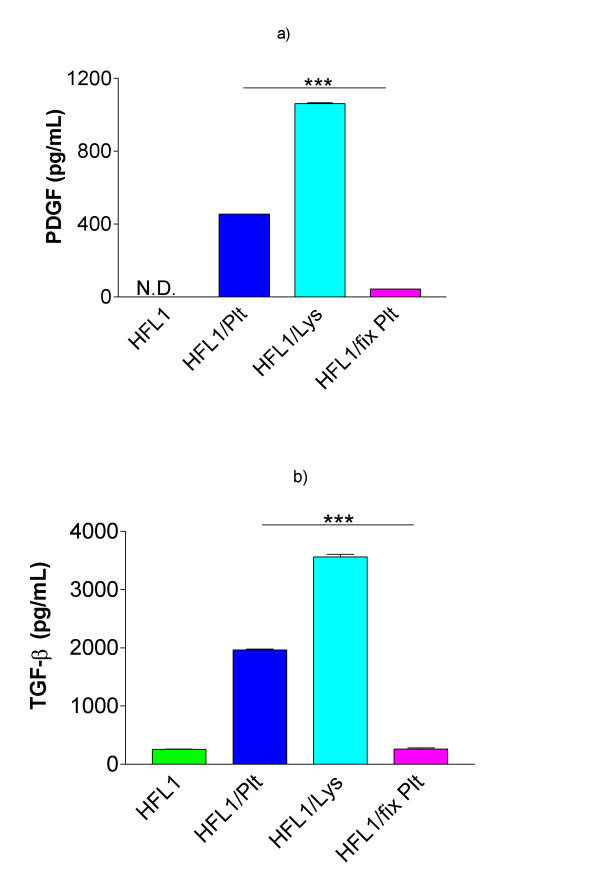
Release of PDGF-AB and TGF-β1 in co-culture. Fibroblasts (HFL1) or fibroblasts together with platelets (Plt), fixed platelets (fix Plt) or platelet lysate (Lys) were cast into collagen gels. After two days of culture, the media surrounding the gels were collected and analysed for a) PDGF-AB and b) TGF-β1 (ELISA). Vertical axis: total concentration of PDGF-AB respectively TGF-β1 (pg/mL). Data shown as mean of duplicates ± SD. *** P < 0.001.
Statistical methods
Data are presented as mean of three replica gels for each condition and standard error of mean (± SEM). As the batch of RTTC, the number of cell passages and culture conditions can affect the gel contraction, the data shown in each figure are taken from a representative experiment of which each was repeated on multiple occasions. Additional data are presented as mean and standard deviation (± SD). Groups of data were evaluated by analysis of variance (ANOVA). Differences between two groups of data were analysed by student's t-test.
Results
Effects of platelets on fibroblast-mediated contraction of collagen gels
Repeated experiments, containing a co-culture of fibroblasts (3 × 105 cells/mL) and platelets (1 × 108 cells/mL) demonstrated a significant (P < 0.001) augmentation of fibroblast-mediated collagen gel contraction (Table 1). The gel area, when measured on four consecutive days, revealed that the augmentation of contraction was time dependent (Figure 1). Thus, gels containing fibroblasts and platelets contracted more at each single point than control gels, containing only fibroblasts. The floating gels contracted most during the first 48 hours. After two days, only a small additional contraction was observed suggesting that the gels have reached a final size. Platelets alone did not induce any contraction.
Table 1.
Platelets augment fibroblast-mediated contraction of collagen gels.
| Cells in collagen gels | Area of gels (% of initial gel area) | SEM | n | |
| Fibroblasts | 46.1 | ± 1.8 | 10 | |
| Platelets | 98.5*** | ± 1.1 | 8 | |
| Fibroblasts/Platelets | 28.4*** | ± 2.6 | 10 |
Area was determined after 2 days of culture. Concentration of fibroblasts and platelets was 3 × 105 respective 108 cells/mL gel. Values are means ± SEM, each including triplicate of gels, n= number of individual experiments. *** P < 0.001 vs. fibroblasts.
Figure 1.
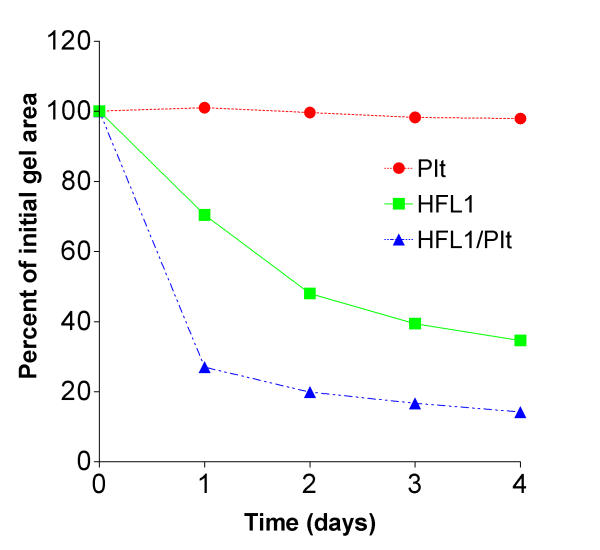
Time dependent augmentation of fibroblast-mediated collagen gel contraction induced by platelets (Plt). Fibroblasts (HFL1: 3 × 105/mL gel) and Plt (108/mL gel) or the two cell types together (HFL1/Plt) were cast into a 24-well tissue culture plate. The assay was performed by releasing the gels into medium immediately after gelation. The area of each gel was measured by an image analyser on four consecutive days. Vertical axis: gel area expressed as percentage of original gel size. Horizontal axis: time (days). Data are presented as mean of triplicate. Standard errors of mean (± SEM) were less than 1%. P < 0.001: HFL1 vs. HFL1/Plt on day 2.
The augmentation of gel contraction was dependent on the number of platelets added (Figure 2). The lowest concentration of platelets that augmented gel contraction was 1 × 107 platelets/mL gel. Adding more than 1 × 108 platelets/mL gel did not result in any additional augmentation of contraction (data not shown).
Figure 2.
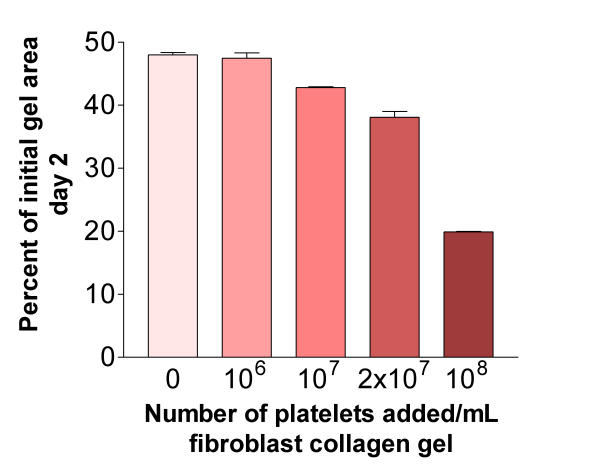
Concentration dependent augmentation of fibroblast-mediated collagen gel contraction. Increasing numbers of platelets were mixed together with fibroblasts in a three-dimensional collagen gel. After gelation, the gels were released and the area of the floating gels was measured after 48 hours of culture. Vertical axis: gel area expressed as percentage of original gel size. Horizontal axis: number of platelets added to gels. Data shown as mean of triplicate ± SEM. P < 0.001: 0 (HFL1) vs. 107 at all data points.
Effects of platelet fixation and platelet lysate on fibroblast-mediated contraction of collagen gels
To evaluate if the platelet-induced augmentation of fibroblast-mediated collagen gel contraction was due to the presence of the platelet structure per se, platelets were fixed with paraformaldehyde and tested in the gel contraction assay.
Fixed platelets co-cultured with fibroblasts did not augment gel contraction compared to gels cultured with fibroblasts and non-fixed platelets (Figure 3). This indicates that viable functional platelets are required to stimulate fibroblast-mediated collagen gel contraction, either by cell-cell contact or by releasing soluble factors in the co-culture system. To test the hypothesis, whether the effect was mediated by platelet associated soluble factors, supernatant from lysed platelets was tested for gel contraction. The lysate stimulated the gel contraction in a time and concentration dependent manner, suggesting that cell-cell contact is not mandatory (Figure 4).
Figure 3.
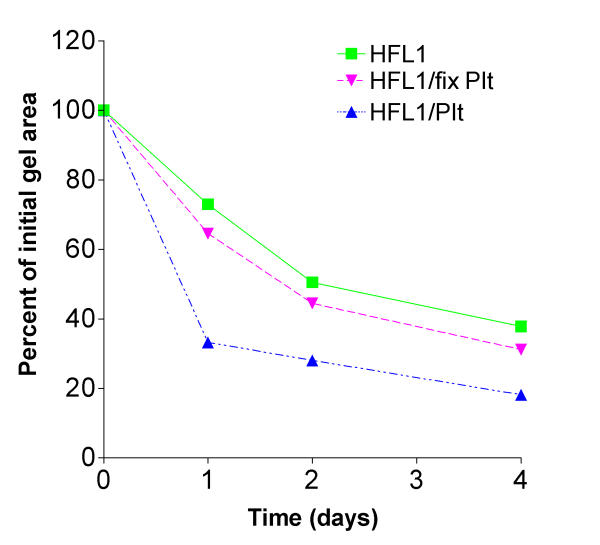
Fixed platelets do not augment fibroblast-mediated collagen gel contraction. Fibroblasts, platelets, fixed platelets (PFA 10 min) or combinations of cell types were cast into collagen gels. The gels were released into medium and the area was measured on four consecutive days. Vertical axis: gel area expressed as percentage of original gel size. Horizontal axis: time (days). Data shown as mean of triplicate. ± SEM were less than 1%. P < 0.001: HFL1/Plt vs. HFL1/fix Plt at all data points.
Figure 4.
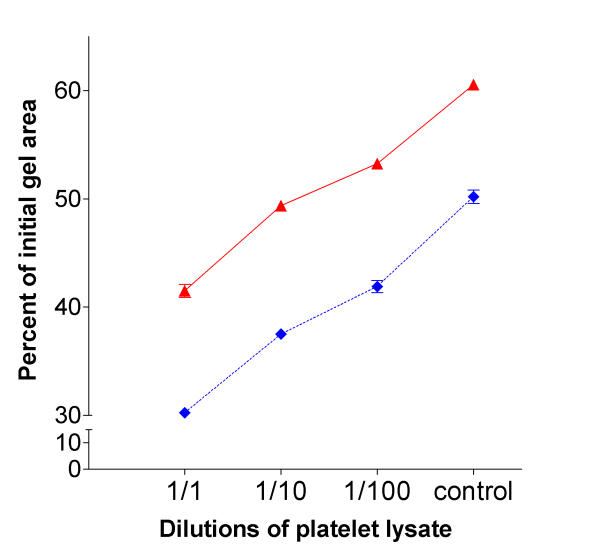
Time and concentration dependent effect of platelet lysate on fibroblast mediated collagen gel contraction. Various dilutions of platelet lysate were mixed together with fibroblasts in collagen gels. After gelation, the gels were released and the area of the floating gels was measured after 2 (solid line) and 4 (dotted line) days. Vertical axis: gel area expressed as percentage of original gel size. Horizontal axis: dilutions of platelet lysate, control contain no lysate. Data presented as mean of triplicate. ± SEM were less than 1%. P < 0.001: HFL1 vs. platelet lysate at all data points.
DNA content
The amount of DNA in collagen gels was determined to assess whether the increased fibroblast-mediated contraction induced by platelets was due to cell proliferation in the culture system. Both co-cultures of whole platelets and platelet lysate resulted in a significant increase in DNA content compared to fibroblasts alone after two days of culture (OD-value: 240.1 ± 41.0 (mean ± SD) and 249.6 ± 19.3 vs. 176.6 ± 15.1; P < 0.01 and P < 0.001 respectively, Figure 5).
Figure 5.
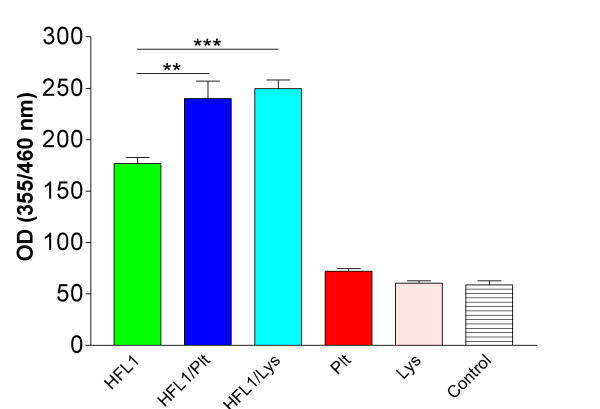
Cell numbers in collagen gels estimated by DNA content. Floating collagen gels containing 300 000 fibroblasts/mL gel (HFL1), HFL1 and 108 platelets/mL gel (HFL1/Plt) or fibroblasts and undiluted platelet lysate, (HFL1/Lys) were cultured for two days. The gels were then dissolved in collagenase and DNA contents in pellets were determined by a fluorometric assay. Control is collagen gels without cells or lysate. Vertical axis: Optical Density (OD) measured at wavelength 355/460 nm. Data shown as means ± SD (n = 6). ** P < 0.01, *** P < 0.001.
According to a standard curve (data not shown), this increase in DNA content equals an increase in cell number from 270 000 cells/mL gel to approximately 450 000 cells/mL gel. To evaluate to what extent this increase in cell number augments gel contraction, various numbers of fibroblasts embedded in collagen gels were assayed for contraction. As shown in Figure 6, and as previously reported by Bell et al. [6], there was a dose dependent increase in fibroblast-mediated collagen gel contraction. According to the platelet-induced proliferation in our system (see Figure 5), not more than 10–15 percent augmentation of fibroblast-mediated collagen gel contraction could be explained by an increased cell number.
Figure 6.
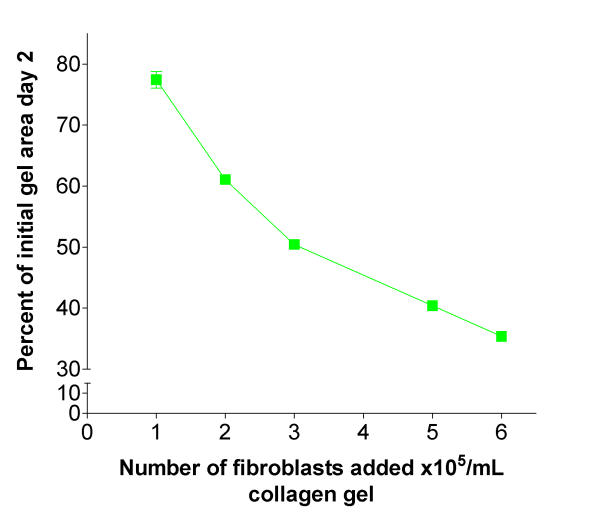
Fibroblast-mediated collagen gel contraction is dependent of cell concentration. Increasing number of fibroblasts (HFL1) were added to three-dimensional collagen gels. After gelation, the gels were released and the area of the floating gels was measured after 48 h of culture. Vertical axis: gel area expressed as percentage of original gel size. Horizontal axis: number of fibroblasts added to gels. Data shown as mean of gel triplicate and ± SEM.
Concentration of PDGF and TGF-β
Both PDGF and TGF-β can augment fibroblast-mediated collagen gel contraction. Therefore, we assayed culture supernatant for these mediators by ELISA. First, the concentrations of cytokines in all culture conditions were determined after two days of culture. The concentration of PDGF-AB in surrounding media in samples containing only fibroblasts, was not detectable (Figure 7a), while it was increased in fibroblast/platelet and fibroblast/platelet lysate co-cultures (454 ± 13 pg/mL (mean ± SD) and 1061 ± 6 pg/mL respectively; P < 0.001). The concentration of PDGF-AB in co-culture of fixed platelets and fibroblasts was significantly lower (44 ± 1 pg/mL; P < 0.001) than in fibroblast/platelet co-culture.
In samples with fibroblasts or fibroblasts and fixed platelets the concentration of TGF-β1 was 258 ± 7 pg/mL (mean ± SD) after two days of culture (Figure 7b). When fibroblasts and unfixed platelets were co-cultured, the concentration of TGF-β1 was 1962 ± 21 pg/mL (P < 0.001 vs. fibroblast and fibroblast/fixed platelets). The concentration of TGF-β was further increased (3561 ± 60 pg/mL) when fibroblasts were cultured with platelet lysate.
In order to investigate the time course of PDGF and TGF-β1 release in fibroblast/platelet co-culture, media from additional experiments were collected after 2, 12, 24 and 48 hours. PDGF-AB was released in higher concentrations at 24 hours compared to PDGF-AA (444 ± 4 pg/mL vs. 257 ± 7 pg/mL, Figure 8). After 48 hours the PDGF release was lower for both isoforms (167 ± 7 pg/mL and 115 ± 21 pg/mL, respectively). There were no detectable levels of PDGF when fibroblasts were cultured alone (data not shown). In contrast, TGF-β1-release increased with increasing length of culture. At 48 hours the concentration was 1888 ± 18 pg/mL in co-culture, while fibroblasts alone released almost the same amount (665 ± 9 pg/mL) at all measured time points (Figure 9).
Figure 8.
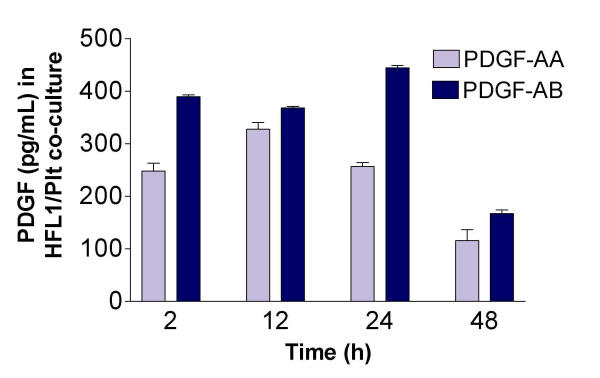
Time-dependent release of PDGF-AA and -AB in fibroblast/platelet co-culture. Fibroblasts (HFL1) together with platelets (Plt) were cast into collagen gels. After 2, 12, 24 or 48 hours of culture, the medium surrounding the gels were collected and analysed for PDGF-AA and PDGF-AB by ELISA. Vertical axis: concentration of PDGF (pg/mL) in medium. Data shown as mean of duplicates ± SD.
Figure 9.
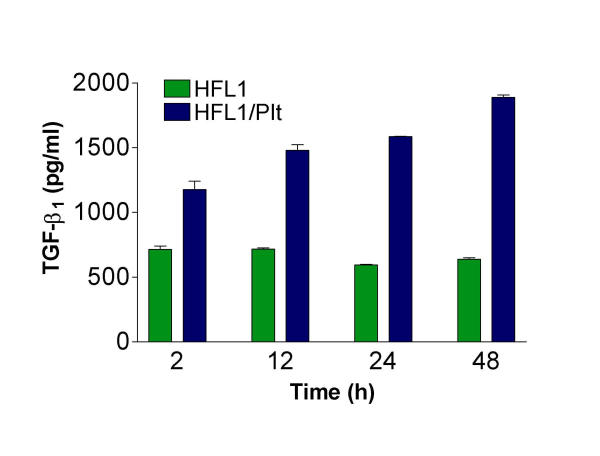
Time-dependent release of TGF-β1 in fibroblast/platelet co-culture. Fibroblasts (HFL1) or fibroblasts together with platelets (Plt) were cast into collagen gels. After 2, 12, 24 or 48 hours of culture, the medium surrounding the gels were collected and analysed for TGF-β1 by ELISA. Vertical axis: concentration of TGF-β1 (pg/mL) in medium. Data shown as mean of duplicates ± SD.
Neutralisation of PDGF and TGF-β bioactivity
To assess the impact of PDGF and TGF-β on platelet-induced augmentation of gel contraction, neutralising antibodies were added to the culture system. Addition of neutralising antibodies to PDGF and TGF-β respectively did partially inhibit the platelet-induced augmentation of gel contraction (Figure 8). When both antibodies were added together an inhibition of 11 percent was observed. Irrelevant antibody (rabbit IgG) did not significantly inhibit the platelet-induced augmentation.
Discussion
In the present study we demonstrate that both whole platelets and platelet lysate can stimulate fibroblast-mediated collagen gel contraction. We were also able to show that the stimulating effect by platelets partially could be attributed to the pro-fibrotic mediators PDGF and TGF-β.
Fibroblasts are believed to be the key cell in tissue remodelling and in the formation of fibrosis. We used an in vitro model for tissue remodelling, fibroblast-mediated contraction of collagen gels, originally described by Bell et al. [6]. Even though the culture system is a simplification of in vivo conditions, regarded as only one aspect of tissue remodelling, the system has been used to elucidate effects of potential fibrotic mediators [16-19]. In the three-dimensional collagen gel system the ability of fibroblasts to contract the gels is dependent on a variety of factors including fibroblast strain, cell density, collagen concentration and the presence of soluble factors [4,9]. For instance, serum is a potent stimulus of fibroblast-mediated collagen gel contraction and has previously been used in a number of studies [20-22].
In the current study we demonstrate that platelets in co-culture with fibroblasts can augment contraction of three-dimensional collagen gels. We found that the stimulation was concentration dependent and that a platelet concentration of 1 × 108 cells/mL gel induced the most pronounced effect. Under normal physiological conditions the platelet count in the bloodstream is about 3 × 108 cells/mL and their lifespan is 7–10 days. In addition, the number of circulating platelets increases significantly during inflammation. Therefore we assumed that the number of platelets present in tissue after injury is probably not less then 108 cells/mL. Two lines of evidence suggest that the stimulatory effect can be mediated by soluble factors. Firstly, fixed platelets had no augmentative effect e.g. the presence of the platelet structure per se did not stimulate contraction. Secondly, a soluble platelet lysate had a pronounced augmentative effect, suggesting that stimulating factors are released from platelets.
Two pro-fibrotic platelet-associated mediators are PDGF and TGF-β and increased concentrations of these mediators have been detected in inflammatory and fibrotic conditions in vivo [23-26]. Since these mediators are known to augment fibroblast-mediated collagen gel contraction [4,9] we sought to determine the relative contribution of them in the contraction assay. PDGF and TGF-β were present in significant levels in culture supernatants and adding neutralising antibodies resulted in a partial inhibition of the platelet-induced augmentation.
We also determined two isoforms of PDGF. PDGF-AA is reported to be the most abundant form released from platelets [27]. However, we found more released PDGF-AB than PDGF-AA in fibroblast/platelet co-culture. TGF-β, frequently present in co-culture, has been shown to down regulate the PDGF-α-receptor on human fibroblasts [28,29]. Since PDGF-AA only bind the α-receptor, while PDGF-AB bind both α- and β-receptors we believe that the observed effect on fibroblast-mediated contraction is predominantly a PDGF-AB effect.
The release of PDGF was highest during the first 24 hours of culture and decreased significantly with increasing length of culture. In contrast, TGF-β1 increased with longer culture periods at measured points during the first 48 hours. One plausible explanation for this difference in kinetics is that PDGF is only released from platelets, while TGF-β1 also can be produced by fibroblasts in response to stimuli during the culture period.
The involvement of these two mediators in the fibroblast-mediated gel contraction assay was confirmed by adding PDGF and TGF-β in the same concentrations as assayed in fibroblast/platelet co-culture, resulting in a small increase of contraction, which could be abolished by neutralising antibodies. In addition, by using excess of antibodies to PDGF and TGF-β we were able to inhibit a part of the platelet-induced contraction. Collectively, these data suggest that PDGF and TGF-β constitute two mediators released from platelets, which partially contribute to the contraction process and that additional platelet-derived mediators or mechanisms can not be excluded.
One possible mechanism involved is cell proliferation. PDGF has a number of effects on mesenchymal cells including stimulation of proliferation [12,30]. Since an altered cell concentration can influence collagen gel contraction [6] we assayed DNA as measure for cell number. The small amounts of DNA in the control, platelet and platelet lysate gels may be DNA fragments originating from the collagen preparation. The increased amount of DNA found when platelets and platelet lysate were cultured together with fibroblasts may in our assay explain approximately 10–15 percent of the increased contraction. However, not even the additive effect of PDGF, TGF-β and proliferation can fully explain the total stimulation induced by platelets. Other mechanisms such as up-regulation of integrin receptors, altered fibroblast phenotype and production/secretion of other stimulating mediators must therefore be considered.
Platelets are a rich source of potential pro-fibrotic mediators [1] that may have a potential to interact with fibroblasts in our in vitro model. For instance, Mio et al. [31] recently showed that lysophosphatidic acid, LPA, which is released from activated platelets, can augment fibroblast-mediated gel contraction. Another inflammatory mediator released by platelets is epidermal growth factor (EGF), a mesenchymal mitogen that stimulate excess deposition of collagen [8]. Thus, a number of platelet-associated mediators are likely to contribute to the pro-fibrotic effect observed in the current study. Whether these factors are directly, or through interaction, involving multiple mediators and/or cells are only speculations. Nevertheless our data point out that platelets accumulated in the lung may be pivotal for the imbalance between inflammatory and fibrotic cytokines thus promoting the subsequent fibrotic process.
Conclusion
We conclude that platelets have the potential to stimulate remodelling of extracellular matrix in vitro and that PDGF and TGF-β may mediate part of this effect. This may be an important mechanism in regulating repair processes after injury. We therefore suggest that platelet/mesenchymal cell interactions may be one important mechanism leading to pulmonary fibrosis.
Abbreviations
ARDS acute respiratory distress syndrome
DNA deoxyribonucleic acid
D-MEM dulbecco's modified eagle medium
EDTA ethylenediamine tetra-acetic acid
ELISA enzyme-linked immunosorbent assay
FBS fetal bovine serum
HFL1 human foetal lung fibroblasts
IgG immunoglobulin G
LPA lysophosphatidic acid
OD optical density
PBS phosphate-buffered saline
PDGF platelet-derived growth factor
PFA paraformaldehyde
Plt platelets
TGF-β transforming growth factor-β
TNE tris-NaCl-EDTA
RTTC rat-tail tendon collagen
SD standard deviation
SEM standard error of mean
Figure 10.
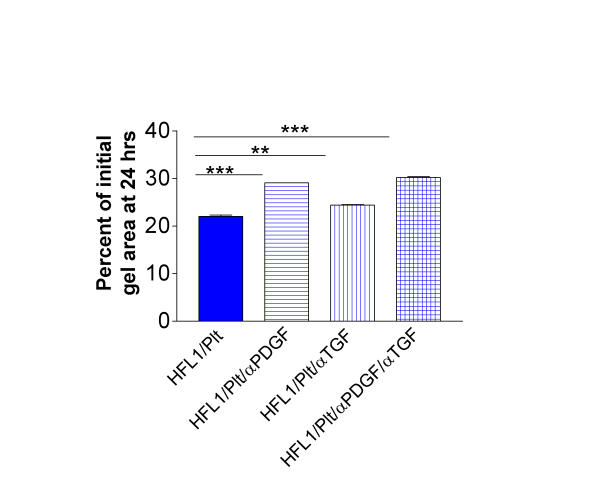
Antibodies to PDGF (αPDGF) and TGF-β (αTGF) partially block platelet-induced stimulation of fibroblast-mediated collagen gel contraction. Collagen gels containing fibroblasts (HFL1) or fibroblasts and platelets (Plt) with or without antibodies were prepared. After gelation, the gels were released into media containing either antibodies to PDGF, TGF-β or the antibodies together. After 24 hours of culture the gel area was measured by an image analyser. Vertical axis: gel area expressed as percentage of initial gel area. Data presented as mean of triplicates ± SEM. ** P <0.01, *** P < 0.001.
Acknowledgments
Acknowledgements
The authors would like to thank Professor Rolf Lewensohn at the Cancer Centrum Karolinska, for letting us use equipment for DNA analysis. The study was supported by grants from the Swedish Heart-Lung Foundation, The Cancer and Allergy Foundation, The Swedish Foundation for Health Care Science and Allergy Research and Karolinska Institutet.
Contributor Information
Ulrika Zagai, Email: ulrika.zagai@ks.se.
Karin Fredriksson, Email: karin.fredriksson@ks.se.
Stephen I Rennard, Email: ulrika.zagai@ks.se.
Joachim Lundahl, Email: joachim.lundahl@ks.se.
C Magnus Sköld, Email: magnus.skold@ks.se.
References
- Heffner JE, Repine JE. Platelets. In: Crystal RG and West JB, editor. The Lung: Scientific Foundations. 2. Philadelphia, Lippincott-Raven Publishers; 1997. pp. 947–959. [Google Scholar]
- Ganong WF. Review of medical physiology. 18. Stamford, Connecticut, Appleton & Lange; 1997. pp. 586–601. [Google Scholar]
- Sime PJ, Tremblay GM, Xing Z, Sarnstrand BO, Gauldie J. Asthma. In: Barnes PJ, Grunstein MM, Leff AR and Woolcock AJ, editor. Interstitial and bronchial fibroblasts. Philadelphia, Lippincott-Raven Publishers; 1997. pp. 475–489. [Google Scholar]
- Montesano R, Orci L. Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci U S A. 1988;85:4894–4897. doi: 10.1073/pnas.85.13.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA. Regulation of fibroplasia in cutaneous wound repair. Am J Med Sci. 1993;306:42–48. doi: 10.1097/00000441-199307000-00011. [DOI] [PubMed] [Google Scholar]
- Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasleton PS, Roberts TE. Adult respiratory distress syndrome - an update. Histopathology. 1999;34:285–294. doi: 10.1046/j.1365-2559.1999.00700.x. [DOI] [PubMed] [Google Scholar]
- Gullberg D, Tingstrom A, Thuresson AC, Olsson L, Terracio L, Borg TK, Rubin K. Beta 1 integrin-mediated collagen gel contraction is stimulated by PDGF. Exp Cell Res. 1990;186:264–272. doi: 10.1016/0014-4827(90)90305-t. [DOI] [PubMed] [Google Scholar]
- Ikuno Y, Kazlauskas A. TGFbeta1-dependent contraction of fibroblasts is mediated by the PDGFalpha receptor. Invest Ophthalmol Vis Sci. 2002;43:41–46. [PubMed] [Google Scholar]
- Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mio T, Adachi Y, Romberger DJ, Ertl RF, Rennard SI. Regulation of fibroblast proliferation in three-dimensional collagen gel matrix. In Vitro Cell Dev Biol Anim. 1996;32:427–433. doi: 10.1007/BF02723005. [DOI] [PubMed] [Google Scholar]
- Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Liu X, Umino T, Cano M, Ertl R, Veys T, Spurzem J, Romberger D, Rennard SI. Human bronchial epithelial cells can contract type I collagen gels. Am J Physiol. 1998;274:L58–65. doi: 10.1152/ajplung.1998.274.1.L58. [DOI] [PubMed] [Google Scholar]
- Kim H, Liu X, Kobayashi T, Kohyama T, Wen FQ, Romberger DJ, Conner H, Gilmour PS, Donaldson K, MacNee W, Rennard SI. Ultrafine carbon black particles inhibit human lung fibroblast-mediated collagen gel contraction. Am J Respir Cell Mol Biol. 2003;28:111–121. doi: 10.1165/rcmb.4796. [DOI] [PubMed] [Google Scholar]
- Fukamizu H, Grinnell F. Spatial organization of extracellular matrix and fibroblast activity: effects of serum, transforming growth factor beta, and fibronectin. Exp Cell Res. 1990;190:276–282. doi: 10.1016/0014-4827(90)90197-i. [DOI] [PubMed] [Google Scholar]
- Adachi Y, Mio T, Takigawa K, Striz I, Romberger DJ, Spurzem JR, Rennard SI. Fibronectin production by cultured human lung fibroblasts in three-dimensional collagen gel culture. In Vitro Cell Dev Biol Anim. 1998;34:203–210. doi: 10.1007/s11626-998-0125-7. [DOI] [PubMed] [Google Scholar]
- Ohga E, Matsuse T, Teramoto S, Ouchi Y. Activin receptors are expressed on human lung fibroblast and activin A facilitates fibroblast-mediated collagen gel contraction. Life Sci. 2000;66:1603–1613. doi: 10.1016/s0024-3205(00)00480-x. [DOI] [PubMed] [Google Scholar]
- Mio T, Liu X, Toews ML, Adachi Y, Romberger DJ, Spurzem JR, Rennard SI. Bradykinin augments fibroblast-mediated contraction of released collagen gels. Am J Physiol Lung Cell Mol Physiol. 2001;281:L164–71. doi: 10.1152/ajplung.2001.281.1.L164. [DOI] [PubMed] [Google Scholar]
- Guidry C. Fibroblast contraction of collagen gels requires activation of protein kinase C. J Cell Physiol. 1993;155:358–367. doi: 10.1002/jcp.1041550217. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Gharaee-Kermani M, Zhang K, Karmiol S, Phan SH. Lung fibroblast alpha-smooth muscle actin expression and contractile phenotype in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1996;148:527–537. [PMC free article] [PubMed] [Google Scholar]
- Lijnen P, Petrov V, Fagard R. In vitro assay of collagen gel contraction by cardiac fibroblasts in serum-free conditions. Methods Find Exp Clin Pharmacol. 2001;23:377–382. doi: 10.1358/mf.2001.23.7.662122. [DOI] [PubMed] [Google Scholar]
- Denis M. Neutralization of transforming growth factor-beta 1 in a mouse model of immune-induced lung fibrosis. Immunology. 1994;82:584–590. [PMC free article] [PubMed] [Google Scholar]
- Bergmann M, Tiroke A, Schafer H, Barth J, Haverich A. Gene expression of profibrotic mediators in bronchiolitis obliterans syndrome after lung transplantation. Scand Cardiovasc J. 1998;32:97–103. doi: 10.1080/14017439850140247. [DOI] [PubMed] [Google Scholar]
- Haque MF, Harris M, Meghji S, Barrett AW. Immunolocalization of cytokines and growth factors in oral submucous fibrosis. Cytokine. 1998;10:713–719. doi: 10.1006/cyto.1997.0342. [DOI] [PubMed] [Google Scholar]
- Hoyle GW, Li J, Finkelstein JB, Eisenberg T, Liu JY, Lasky JA, Athas G, Morris GF, Brody AR. Emphysematous lesions, inflammation, and fibrosis in the lungs of transgenic mice overexpressing platelet-derived growth factor. Am J Pathol. 1999;154:1763–1775. doi: 10.1016/S0002-9440(10)65432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma Y, Dvonch V, Grotendorst GR. Platelet-derived growth factor AA homodimer is the predominant isoform in human platelets and acute human wound fluid. Faseb J. 1992;6:2996–3001. doi: 10.1096/fasebj.6.11.1644262. [DOI] [PubMed] [Google Scholar]
- Bonner JC, Badgett A, Lindroos PM, Osornio-Vargas AR. Transforming growth factor beta 1 downregulates the platelet-derived growth factor alpha-receptor subtype on human lung fibroblasts in vitro. Am J Respir Cell Mol Biol. 1995;13:496–505. doi: 10.1165/ajrcmb.13.4.7546780. [DOI] [PubMed] [Google Scholar]
- Soma Y, Mizoguchi M, Yamane K, Yazawa N, Kubo M, Ihn H, Kikuchi K, Tamaki K. Specific inhibition of human skin fibroblast chemotaxis to platelet-derived growth factor A-chain homodimer by transforming growth factor-beta1. Arch Dermatol Res. 2002;293:609–613. doi: 10.1007/s00403-001-0279-6. [DOI] [PubMed] [Google Scholar]
- Price WA. PDGF-BB regulates IGF-mediated IGFBP-4 proteolysis in fetal lung fibroblasts. Exp Lung Res. 2001;27:655–674. doi: 10.1080/019021401317138469. [DOI] [PubMed] [Google Scholar]
- Mio T, Liu X, Toews ML, Rennard SI. Lysophosphatidic acid augments fibroblast-mediated contraction of released collagen gels. J Lab Clin Med. 2002;139:20–27. doi: 10.1067/mlc.2002.120650. [DOI] [PubMed] [Google Scholar]


