Abstract
A self-propelling colonoscopic device moving inside the colonic tube should be able to periodically grip safely to the colonic wall as well as to manipulate the generated friction. The feasibility of achieving high grip and friction manipulation by covering the device with mucoadhesive films is experimentally tested. More precisely, the frictional behaviour of mucoadhesive films inside the colonic tube is tested in vitro in porcine colon. It appears that mucoadhesive films generate significantly higher friction than conventional materials (ANOVA p=0, 95% CIs=−3.04, −2.14). The geometry of the film plays a role as well. When holes are, for instance, present in the film geometry and are large enough so that the colonic tissue can wrap their borders, friction can be significantly increased (ANOVA p=0, 95% CIs=−2.53, −1.26). By altering the contact area or the film geometry, friction manipulation can be achieved. Moreover, a simple theoretical model is developed and experimentally verified (R=0.92). The model can be used to estimate the level of the friction generated by three-dimensional configurations of mucoadhesive films as a function of their geometric characteristics and the material properties of the colon.
Keywords: colonoscopy, colonoscopic device, friction, mucoadhesives
1. Introduction
When a patient shows symptoms of colonic disease, a colonoscopy is carried out: a standard medical procedure in which a flexible endoscope is pushed through the colon for visual inspection of the colonic wall and for simple interventions. Although colonoscopy is considered to be a relatively safe procedure, it entails a 0.2% risk of perforation of the colonic wall (Anderson et al. 2000; Kavic & Basson 2001) and is usually accompanied by painful cramps and often by incidences of incomplete inspection. Those drawbacks of conventional colonoscopy indicate the need to develop alternative self-propelling intestine inspection and intervention devices that are able to be pulled from ahead instead of being pushed from behind like the conventional flexible endoscopes. The main challenge for the development of such devices is their locomotion along the colonic surface, with which they should generate sufficient friction for grip.
At the TU Delft, a new method to increase friction with the colonic surface by means of mucoadhesive films is being investigated (Dodou et al. 2005a). In vitro experiments showed that, when the device pads are covered with mucoadhesive films, significantly high friction can be generated, ensuring grip to the colonic surface (Dodou et al. 2006). Moreover, it was shown that the friction of the films depends strongly on their size (Dodou et al. 2005b) as well as geometry (Dodou et al. 2006). During these in vitro experiments, a piece of porcine colon was extracted, opened longitudinally and fixed on a heating pad with the inner surface up. Then a plate with a mucoadhesive film was laid on the tissue, loaded with a weight simulating the intra-abdominal pressure and pulled horizontally by means of a tensile testing machine. The advantage of carrying out the experiments on an open colonic segment was that one could observe more and interfere more easily. The intestine inspection and intervention devices, however, will ultimately move through the colonic tube and not on the surface of an open colonic segment. The behaviour of the mucoadhesive films should therefore be tested in vitro inside a colonic tube.
2. Experimental set-up
When measuring friction inside a colonic tube, a number of challenges are encountered, such as the limited accessibility as well as the lack of ability and need to track visually the progress of the measurement. The intra-abdominal pressure should be simulated so that the in vitro testing conditions resemble the environment inside the abdomen. It should be noted that there are already some commercially available training models for colonoscopy, ranging from very simple up to highly advanced and sophisticated. The simple models are mainly built to train manoeuvres required during a colonoscopy. These models are not sufficiently realistic to test the performance of self-propelling devices inside the colon. The advanced models (colonoscopy simulators) are suitable only for conventional colonoscopes and not for new experimental devices. Since the existing models were not suitable for our measurements, an experimental set-up has been built that allows us to measure the friction generated by mucoadhesive films inside the colonic tube and tracks visually their static and dynamic behaviour.
The set-up consists of a transparent box (figure 1) of size 40×30×20 cm. Two opposite sides of the box contain a hole with a closable tube to which a colonic segment can be attached. Considering that the diameter of the colon varies along its length (unstretched pig colon diameter ranges between 25 and 40 mm), the diameter of the closable tubes should be adjustable as well (20–35 mm). The intra-abdominal pressure, varying between 0.67 and 0.93 kPa (Kozarek et al. 1980; Sanchez et al. 2001), is simulated by filling the box with water. To simulate an average intra-abdominal pressure of 0.8 kPa, the box is filled with water up to 8 cm above the level of the colonic segment. To avoid accidental entry of water into the colonic segment, the two ends of the segment are tightly fixed to the holes by means of rubber rings. An extra closable hole at one of the bottom corners serves as an outlet for the water after the use of the set-up.
Figure 1.
Experimental set-up for testing the friction of mucoadhesive films inside a colonic tube.
For each measurement, a mucoadhesive film was fixed on a test tube, which was acting as the inspection device, and brought inside the colon. The test tube was connected via a thread and a pulley to the force sensor of a tensile testing machine (Zwick 1484). The tensile testing machine pulled the tube forward with constant speed (60 mm min−1) and recorded the trace of the generated friction force.
From previous experiments it was known that, although mucoadhesive films stick to the colon with considerably high static friction, they lose their sticking ability as soon as they have been sheared a few centimetres along the colonic surface (Dodou et al. 2005b). Moreover, as soon as the films unstick, film fragments often remain behind the colonic surface, eventually influencing its properties. For these reasons, it was critical for each measurement to protect the mucoadhesive film from premature contact with the colonic surface before the test tube had arrived at an unused piece of the colonic segment. To achieve that, the test tube was enclosed inside a glass pipe that was inserted into the colon through one of the holes in the box. At the same time, the thread of the test tube was passed through the other hole with the help of a fishing rod and connected to the force sensor of the tensile testing machine. As soon as the test tube reached an unused piece of the colonic segment, the glass pipe was retracted, bringing the test tube in contact with the colonic surface.
After the test tube had been brought into position inside the colonic segment, a laparoscopic camera was inserted through one of the closable tubes, to keep visual control of the measurement. The laparoscopic camera was connected to a light source, a monitor and a video recorder, and it was able to follow and record the behaviour of the test tube during the measurement. The laparoscopic camera was enclosed in a glass pipe. The glass pipe served to open the colon, which tended to close under the water pressure, and ensured a minimum monitoring distance from the colonic wall.
From the experiments on an open colonic segment, it was known that the contact area of the mucoadhesive film with the colonic surface is crucial for the generated friction (Dodou et al. 2005b). This means that a device (or the currently used test tube) gripping the colon should maximize contact with the periphery of the colonic wall. The diameter of the colon on the other hand varies along its length whereas the axial stiffness of the colonic tube is high. A test tube with diameter larger than that of the colonic tube would eventually not succeed in stretching the colonic tube without damaging it and pass through. A test tube with diameter smaller than that of the colonic tube would create significantly reduced contact with the colonic wall (figure 2). The diameter of the test tube should be therefore adjustable in order to consistently achieve maximum contact with the colonic wall. For this reason, test tubes of three different diameters were prepared and the tube with the most suitable diameter for each colonic segment was selected for every measurement.
Figure 2.
Test tube with diameter smaller than the diameter of the colonic tube. The contact area is not maximized.
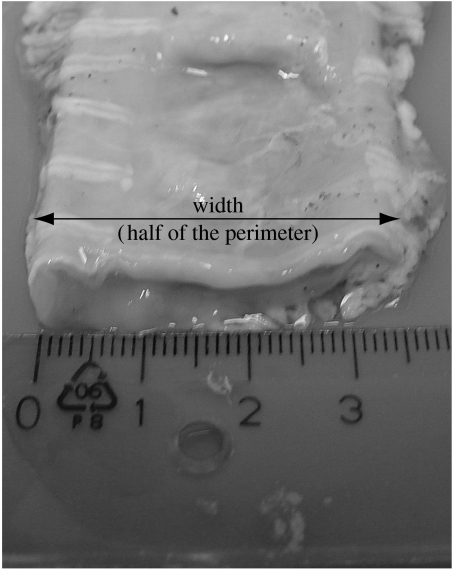
To determine the diameters of the test tubes, we measured the diameter along three porcine colons extracted from animals of similar weight (30–40 kg). Each colon was cut into 10 cm long segments and laid on a horizontal surface (figure 3). The width of each segment was then measured and found to range between 25 mm close to the rectum and up to 40 mm close to the caecum. Since the width is equal to half of the colon perimeter, it was easily calculated that the diameter of the colon varied in average between 15 and 25 mm. Based on these measurements, it was decided to prepare test tubes with inner diameters of 15, 20 and 25 mm and use the most suitable size according to the diameter of each colonic segment fixed in the box.
Figure 3.
Defining the diameter of the colonic tube.
The preparation of the mucoadhesive films is described elsewhere (Dodou et al. 2006). All animal procedures were performed using institutionally approved protocols.
3. Experiment 1
The aim of experiment 1 was to investigate in vitro whether and to what extent a mucoadhesive film can increase the friction inside the colon. The role of the film geometry was investigated as well.
3.1 Measuring protocol
From the experiments on an open colonic segment, it was found that the area of the film, the presence of holes as well as a long borderline are of importance to generate high friction (Dodou et al. 2006). To test whether those parameters are crucial inside a colonic tube as well, three different geometric configurations of mucoadhesive films with the same surface area (1.3×103 mm2) were tested (table 1, geometries 2–4). A test tube without mucoadhesive film was tested as a reference (geometry 1). All test tubes were tested in one animal, using three colonic segments per tube. For all geometries, test tubes of all three diameters were available. For every measurement, the tube with the most suitable diameter for each colonic segment was selected and tested. The length of the film and the test tube was adjusted so that the area of the film was always the same and equal to 1.3×103 mm2.
Table 1.
Geometric configurations of mucoadhesive films fixed to test tubes tested in experiment 1. (Dimensions are in mm.)
| diameter of test tubes (mm) | |||
|---|---|---|---|
| 15 | 20 | 25 | |
| geometries | |||
| 1 |  |
 |
 |
| 2 |  |
 |
 |
| 3 |  |
 |
 |
| 4 |  |
 |
 |
| film area (mm2) | 1.3×103 | 1.3×103 | 1.3×103 |
3.2 Results
The results showed that the static friction generated by mucoadhesive films on the colonic surface is considerably higher than the friction generated by the reference test tube (ANOVA p=0.02, 95% CIs=−4.8, −0.4; figure 4). The geometry of the film, however, did not appear to play a significant role (ANOVA p=0.55), contradicting the results derived from measurements on an open colonic segment.
Figure 4.
Experimental data of the results of experiment 1 showing the maximum static friction force between the colonic surface and the mucoadhesive films of varying geometric configurations. Diameter of test tube: circles, 15 mm; diamonds, 20 mm; squares, 25 mm.
When testing on an open colonic segment, the mucoadhesive film is fixed at the bottom of a plate lying on the colonic surface. The role of the film geometry is due to the fact that the plate with the film sinks into the soft colonic tissue and therefore the borders of the film contribute to the generated friction force. In other words, the colonic tissue wraps around the borders of the plate with the film (figure 5a). The longer the borderline, the stronger its role in the friction. The same occurs with holes: when holes are present in the film geometry, they are opened through the plate so that the tissue is able to wrap around the hole borders (Dodou et al. 2006).
Figure 5.
Testing on an (a) open colonic segment and (b) inside the colonic tube during experiment 1. When testing on an open colonic segment, the holes of the geometry are open through the mucoadhesive film and the plate. The colonic tissue wraps around the film borders at the front and back of the plate as well as around the holes, contributing to friction. When testing inside the colonic tube during experiment 1, the holes are limited up to the film thickness, thus strongly decreasing the effect of the borderline. Only the film border at the back contributes thus to the friction.
In experiment 1, the holes are not opened through the tube walls but only up to the thickness of the film. The colonic tissue can therefore only wrap around the film border at the back of the tube and not at the front of the tube or in a hole (figure 5b). The effect of the borderline is thus very small.
Figure 4 shows that the size of the test tube used in each measurement is of importance and that, for every geometry, a test tube with diameter 25 mm generates higher friction than that with diameter 15 mm. These results can be explained once more by the role of the borderline: for a given contact area, a larger test tube has a longer borderline and it is expected to generate higher friction.
4. Experiment 2
The aim of experiment 2 was to investigate the role of geometry when the holes are opened throughout the mucoadhesive film and the test tube, so that the colonic tissue can easily wrap around the borders of the film and the tube and thus increase friction.
4.1 Measuring protocol
Three different geometric configurations of mucoadhesive films were tested (table 2). A test tube without mucoadhesive film was tested as well as being used as a reference (geometry 1). All four cases were tested in two animals, using six colonic segments per tube. Since eventual differences between the colons tested in experiments 1 and 2 could lead to misinterpretation of the results, geometry 2 from experiment 1 was tested again in the colon used for experiment 2. With geometry 5, we aimed to test a case in which the front borderline of the film can contribute to the generated friction. Geometry 6 is similar to geometry 4, with the only difference being that the holes in geometry 6 are opened throughout the tube. For all geometries, the test tubes of all three diameters were available. For every measurement, the tube with the most suitable diameter for each colonic segment was selected and tested. The length of the film and the test tube was adjusted so that the area of the film was always the same and equal to 1.3×103 mm2.
Table 2.
Geometric configurations of mucoadhesive films fixed to test tubes tested in experiment 2. (Dimensions are in mm.)
| diameter of test tubes (mm) | |||
|---|---|---|---|
| 15 | 20 | 25 | |
| geometries | |||
| 1 |  |
 |
 |
| 2 |  |
 |
 |
| 5 |  |
 |
 |
| 6 | 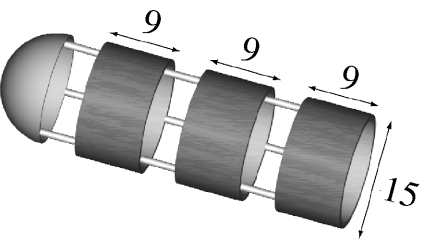 |
 |
 |
| film area (mm2) | 1.3×103 | 1.3×103 | 1.3×103 |
4.2 Results
The results showed that the friction generated by mucoadhesive films on the colonic surface is significantly higher than that generated by the reference test tube (geometry 1 versus geometry 2: ANOVA p=0, 95% CIs=−3.04, −2.14; figure 6). The geometry of the film plays a role as well (geometry 2 versus geometry 5: ANOVA p=0, 95% CIs=−2.53, −1.26; geometry 5 versus geometry 6: ANOVA p=0, 95% CIs=−2.32, −0.94). In all three tested geometries, a part of the generated friction is due to the film area and another part is due to borderline of the film. Since the film area is constant, the differences in the friction generated by the three geometries can be attributed to differences in the film borderline. For instance, in geometry 2, only the film borderline at the back of the tube can play a role in the friction, whereas in geometry 5 the film borderline at the front of the tube can contribute to the friction as well (figure 7). This can explain why geometry 5 generated higher friction than geometry 2. Interestingly, the friction generated by geometry 6 was similar to geometry 2 (geometry 2 versus geometry 6: ANOVA p=0.4, 95% CIs=−0.94, −0.42), despite the extended presence of borderline at the back and front of the tube as well as around the holes. A possible explanation can be that the holes in geometry 6 had a length of 5 mm, whereas the hole in geometry 5 was 10 mm long. Holes of 5 mm are apparently too small and the colonic tissue cannot enter inside them to wrap around the film borderline. The incapability of the colonic tissue to interact with the holes of geometry 6 was observed during the experimental procedure as well, opposite to a strong wrapping around the front film borderline in the case of geometry 5. As a result, only the film borderline at the back of geometry 6 contributes to the friction. In other words, the role of the borderline in geometry 6 is similar to geometry 2 and this explains why the two geometries generated a similar amount of friction. It should be noted that, similar to experiment 1, the size of the tube used in each measurement is also of importance.
Figure 6.
Experimental data of the results of experiment 2 showing the maximum static friction force between the colonic surface and mucoadhesive films of varying geometric configurations. Diameter of test tube: circles, 15 mm; diamonds, 20 mm; squares, 25 mm.
Figure 7.
The role of the film borders for the friction generated by geometries 2 and 5. In (a) geometry 2, only the film borderline at the back of the tube can play a role in the friction. In (b) geometry 5, the film borderline at the front of the tube can contribute to the friction as well.
5. Theoretical model
To describe the friction generated on an open colonic segment by mucoadhesive films of various two-dimensional geometries, we previously developed a simplified theoretical model that expresses the generated friction as a function of the film geometry and the tissue properties (Dodou et al. 2006). The aim of this section is to adjust the theoretical model so that it can predict the friction of three-dimensional film geometries gripping inside the colonic tube.
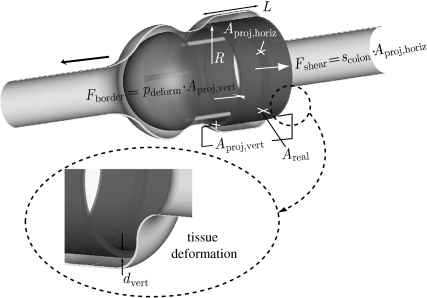
Consider a test tube of radius R with the configuration of geometry 5 (figure 8). The tube is covered with a mucoadhesive film of length L and is inserted into a colonic tissue segment subjected to intra-abdominal pressure. Owing to the intra-abdominal pressure, the colonic tissue wraps around the tube. The friction force Ffric between the film and the colonic tissue can then be expressed as
| (5.1) |
in which Fshear is the resistance to shearing and Fborder is the resistance by the tissue that is wrapped around the borderline of the film. The variable Fshear can be calculated as
| (5.2) |
in which scolon is the shear strength of the colon–mucoadhesive interface and Aproj,horiz is the surface area of the film, i.e.
| (5.3) |
Owing to the intra-abdominal pressure, the tissue wraps the tube at the rear and the front sections of the film. Both sections of the film, which are perpendicular to the direction of shearing, contribute to the generated friction. Hence
| (5.4) |
in which Aproj,vert is the area of the wrapped film at the rear and front sections of the tube and pdeform defines the extent up to which the tissue is wrapped around the borderline of the film. Aproj,vert can be calculated as
| (5.5) |
in which dvert is the height of the (part of the) film that is in contact with the wrapping tissue. It should be noted that the effect of the rear and front sections can differ. This possible difference is not taken into account in this approach for simplicity reasons. For further elaboration, the reader is referred to Dodou et al. (2006). The parameter pdeform is a nonlinear function of the intra-abdominal pressure, the longitudinal stiffness of the colon and the geometric characteristics of the test tube (e.g. length and diameter), i.e.
| (5.6a) |
Initially, we define pdeform as a linear function of the intra-abdominal pressure Pintra and a dimensionless constant ccolon that includes the tissue stiffness and the geometric properties of the test tube, i.e.
| (5.6b) |
It should be noted that (5.6b) is an (over)simplification in order to primarily evaluate to which extent friction can be estimated by means of the theoretical model. Further investigation for a more accurate definition of pdeform should be carried out in the future. By combining equations (5.1), (5.2) and (5.4), the friction force Ffric between the film and the tissue can be expressed as
| (5.7) |
By combining equations (5.3), (5.6b) and (5.7), the friction force can be calculated by means of the tube and film geometric characteristics and the material properties of the tissue, i.e.
| (5.8a) |
A similar relationship can be derived for geometry 2 (table 2). Since in this case there is no film at the front section of the tube, we assume that the wrapping at the front does not contribute significantly to the friction. At the rear section of the tube, wrapping is considered to be of importance for the generated friction. Aproj,vert is thus half of that in the case of geometry 5. Additionally, the influence of the front section of the film is taken into account when defining Adeform, which is half of that in the case of geometry 5 as well. In the case of geometry 2, the friction force can thus be expressed as
| (5.8b) |
Figure 8.
Colonic tissue wrapping in the case of geometry 6. The thick black arrow indicates the direction of shearing.
6. Experimental fitting
In order to check the validity of the model in equations (5.8a) and (5.8b), we used it to calculate the static friction for geometries 2 and 5. We assume that the intra-abdominal pressure is sufficient to cause tissue wrapping up to an extent that dvert is the total thickness of the film. We defined the material parameters scolon and ccolon by using equations (5.8a) and (5.8b) for two of the measurements. The minimum friction value generated by geometry 2 and the maximum friction value generated by geometry 6 were selected. Then, the friction was estimated for all cases by equations (5.8a) and (5.8b) and compared with the measured values (R=0.92; figure 9). The systematic underestimation of the calculated friction in comparison with the measured values may be related to the simplification of the pdeform and urges a more elaborate approach.
Figure 9.
Experimental fitting of friction values measured for all test tubes of geometries 2 and 5 with the calculated friction by equations (5.8a) and (5.8b). The solid line defines the 45° slope.
7. Discussion
7.1 Friction at the front section of the device
In order to gain a better understanding of the frictional behaviour of a self-propelled endoscope, Baek et al. (2004) measured the friction generated inside the small intestine by capsule-shaped endoscopes of various sizes and shapes (the capsules were not covered with mucoadhesive films). Baek et al. concluded that increased contact area contributes to friction and therefore capsules with protrusions along their periphery generate higher friction. It was further concluded that the shape of the front section of the capsule affected the friction more than the mid or the rear section since the front section pushes the wall outward as the capsule moves through the gut. By contrast, in the case of a device (or the currently used test tube) gripping in the colon by means of mucoadhesive films, the friction of the front section does not contribute significantly to the friction. This can be further supported by the very low values of friction generated by geometry 1. In other words, in our case, the friction of the mucoadhesive film is so high that the resistance required to open the colonic tube can be neglected.
As a first design approach, the device can consist of a cylinder with invariable diameter covered with two pads that are coated with mucoadhesive films. Moreover, it will be desirable to introduce holes in the pads so that the generated friction increases owing to the long borderline. Since the holes compensate for size, the device can generate high static friction by meeting at the same time the requirement of compactness. The number of holes as well as their distribution (eccentric or concentric) is a question of future design optimization.
7.2 Friction manipulation by means of hole size
Experiment 2 showed that the size of the holes influences the measured friction. By altering the size of the holes, it seems therefore feasible to switch between high and low friction values. How could we implement this possibility for friction manipulation into the design of an intestine inspection device? As a first approach (figure 10), and alternatively to the design discussed in the previous paragraph, the device can have a configuration similar to geometry 5 (table 2) in which the back section is covered with a mucoadhesive film. The only difference with geometry 5 is that the film is slightly shorter than the back section, so that the rear part of the back section remains uncovered. The wrapping of the colonic tissue around the rear part of the device therefore does not contribute to friction significantly. The front section of the device consists of a low-frictional material, i.e. rubber. The distance between the two sections of the device can be altered on demand. When the device should grip with high friction, the distance between the two sections is kept large, so that the colonic tissue can wrap the front borderline of the film and contribute to friction. When the device should slide, the front section of the device moves backwards, decreasing its distance with the back section. Consequently, the colonic tissue cannot wrap around the front film borderline anymore and the friction decreases. To grip again, the front section moves forward to increase its distance from the back section to allow tissue wrapping, friction increase and grip.
Figure 10.
Design concept of a colonoscopic device that achieves high grip by means of mucoadhesive films. (a) The distance between the front part of the device and the part with the film is large so that the colonic tissue wraps around the front film border and increases friction and grip. (b) The distance between the front part of the device and the part with the film decreases so that the colonic tissue cannot wrap around the front film border anymore. Friction and grip decrease. (c) Friction is low(er) so that the device can unstick and slide a step forward. (d) The distance between the front part of the device and the part with the film increases again so that the colonic tissue wraps around the front film border and increases friction and grip.
It has been shown from previous experiments (Dodou et al. 2006) that the motion of a mucoadhesive film sticking on the colonic surface is initiated by cohesive failure of the film, after which it is not possible for the films to regain their high sticking action. A new film should be used for each of the device steps. This can be achieved by including a refreshing mechanism in the device, which is able to provide a new (piece of) film each time the device requires sticking to the colon. However, to keep the design of the device simple, the changes of the film properties would be preferably reversible, so that one single film could repeatedly switch between the states of stick–unstick–slide. To realize reversible film behaviour, the use of environmentally sensitive polymers with mucoadhesive properties is currently being investigated.
8. Future directions
To achieve a more realistic representation of the abdominal anatomy, we aim to place jelly objects into the box to simulate organs inside the abdomen. Ligament-like attachments can be created by using suture wire or clamps attached to the colon as well as to suction pads, which can be placed anywhere on the inner surface of the box. In this way, frictional behaviour can be tested not only along a straight colonic segment but also within a more realistic anatomic colonic configuration with curves.
Although the experimental fitting of the theoretical model was satisfactory, one should be careful with its interpretation, considering that the number of the geometries used for its verification is limited. Although the model in its two-dimensional form has satisfied a larger number of experimental data (Dodou et al. 2006), the validity of its three-dimensional form should be checked against more three-dimensional geometries.
The in vitro testing showed that mucoadhesive films increase friction inside the colonic tube and the friction depends on the geometry of the films. However, it should be noted here that, although fresh colonic material was used, there are inevitable differences in the condition of in vitro and in vivo mucus. The thickness of the colonic mucous layer in vitro is usually less than the thickness of the mucous layer observed in vivo. According to Mortazavi & Smart (1995), this difference does not play a crucial role in the mucoadhesive performance, since mucoadhesives show adhesive ability even in the complete absence of mucus. Furthermore, colonoscopic procedures are carried out after treatment with laxatives. It has been proven that the use of laxatives leads to reduced formation of mucus (Farthing 2004). It seems thus possible that the reduced presence of mucus on the colonic surface in vitro might compensate for the reduced formation of mucus after the treatment with laxatives in vivo. It should be noted, however, that the physico-chemical properties of the mucus in vitro may differ from that of the in vivo mucous layer. Further discussion about the in vitro/in vivo differences is provided in Dodou (2006). One of the following steps is therefore to test the feasibility of mucoadhesive films as a means for colonic locomotion in vivo as well. For this reason, a mechanical prototype is being built. A major design challenge when testing in vivo is to equip the mechanical prototype with a mechanism of film refreshment, since mucoadhesive films lose their adhesiveness after a single use. The experimental findings of in vivo testing may induce a series of new questions to be answered. Different mucous and colonic wall properties as compared with the in vitro situation might affect the locomotion of the device. The in vivo testing will reveal the real challenges and indicate the potential of sticky walking for a colonoscopic device.
Acknowledgments
This research has been made possible by the Van der Leeuw professorship (Dutch Technology Foundation STW) awarded to one of the authors (P.A.W.). The research of another author (P.B.) has been made possible by a fellowship of the Royal Netherlands Academy of Arts and Sciences.
References
- Anderson M.L, Pasha T.M, Leighton J.A. Endoscopic perforation of the colon: lessons from a 10-year study. Am. J. Gastroenterol. 2000;95:3418–3422. doi: 10.1111/j.1572-0241.2000.03356.x. [DOI] [PubMed] [Google Scholar]
- Baek N.K, Sung I.H, Kim D.E. Frictional resistance characteristics of a capsule inside the intestine for microendoscope design. Proc. Inst. Mech. Eng. Part H: J. Eng. Med. 2004;218:193–201. doi: 10.1243/095441104323118914. [DOI] [PubMed] [Google Scholar]
- Dodou, D. 2006 Colonic locomotion. PhD dissertation, Delft University of Technology.
- Dodou D, Breedveld P, Wieringa P.A. Mucoadhesives in the gastrointestinal tract: revisiting the literature for novel applications. Eur. J. Pharm. Biopharm. 2005a;60:1–16. doi: 10.1016/j.ejpb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Dodou, D., Girard, D., Breedveld, P. & Wieringa, P. A. 2005b Intestinal locomotion by means of mucoadhesive films. In Proc. ICAR '05, 12th International Conference on Advanced Robotics, Seattle, WA, 18–20 July, pp. 352–359. Piscataway, NJ: IEEE.
- Dodou D, Breedveld P, Wieringa P.A. The role of geometry in the friction generated on the colonic surface by mucoadhesive films. J. Appl. Phys. 2006;100:014904. doi: 10.1063/1.2209070. [DOI] [Google Scholar]
- Farthing M.J.G. Treatment options in irritable bowel syndrome. Best Pract. Res. Clin. Gastroenterol. 2004;18:773–786. doi: 10.1016/j.bpg.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Kavic S.M, Basson M.D. Complications of endoscopy. Am. J. Surg. 2001;181:319–332. doi: 10.1016/S0002-9610(01)00589-X. [DOI] [PubMed] [Google Scholar]
- Kozarek R.A, Earnest D.S, Silverstein M.E, Smith R.G. Air-pressure-induced colon injury during diagnostic colonoscopy. Gastroenterology. 1980;78:7–14. [PubMed] [Google Scholar]
- Mortazavi S.A, Smart J.D. An investigation of some factors influencing the in vitro assessment of mucoadhesion. Int. J. Pharm. 1995;116:223–230. doi: 10.1016/0378-5173(94)00299-K. [DOI] [Google Scholar]
- Sanchez N.C, Tenofsky P.L, Dort J.M, Shen L.Y, Helmer S.D, Smith R.S. What is normal intra-abdominal pressure? Am. Surg. 2001;67:243–248. [PubMed] [Google Scholar]