Abstract
Human mesenchymal stem cells (hMSCs) isolated from bone marrow aspirates were cultured on silk scaffolds in rotating bioreactors for three weeks with either chondrogenic or osteogenic medium supplements to engineer cartilage- or bone-like tissue constructs. Osteochondral composites formed from these cartilage and bone constructs were cultured for an additional three weeks in culture medium that was supplemented with chondrogenic factors, supplemented with osteogenic factors or unsupplemented. Progression of cartilage and bone formation and the integration between the two regions were assessed by medical imaging (magnetic resonance imaging and micro-computerized tomography imaging), and by biochemical, histological and mechanical assays. During composite culture (three to six weeks), bone-like tissue formation progressed in all three media to a markedly larger extent than cartilage-like tissue formation. The integration of the constructs was most enhanced in composites cultured in chondrogenic medium. The results suggest that tissue composites with well-mineralized regions and substantially less developed cartilage regions can be generated in vitro by culturing hMSCs on silk scaffolds in bioreactors, that hMSCs have markedly higher capacity for producing engineered bone than engineered cartilage, and that chondrogenic factors play major roles at early stages of bone formation by hMSCs and in the integration of the two tissue constructs into a tissue composite.
Keywords: osteogenesis, chondrogenesis, silk scaffold, bioreactor, osteochondral composite
1. Introduction
Osteoarthritis and other traumatic injuries of load-bearing cartilage and underlying bone often result in a loss of mobility of the joint and severe discomfort. A representative therapy of osteochondral defects is mosaicplasty—the grafting of cartilage using osteochondral plugs from non-load-bearing zones (Bartha et al. 2006). In many cases, this approach suffers from donor site morbidity, limited availability and topological mismatch (Simon & Jackson 2006). Tissue-engineered grafts generated from the patient's own cells and degradable biomaterials would be a viable alternative to mosaicplasty since they can be (i) grown from small initial numbers of cells, (ii) tailored to specific size and shape as well as structural and biomechanical specifications, and (iii) coupled with biochemical cues to enhance tissue integration. A number of meritorious approaches have been proposed to engineer cartilage alone and osteochondral grafts that included the use of a variety of scaffold materials (Schaefer et al. 2000, 2002; Sherwood et al. 2002; Alhadlaq et al. 2004; Tuli et al. 2004), chondrogenic and osteogenic cells (Schaefer et al. 2002; Jiang et al. 2005; Meinel et al. 2006) and regulatory factors (Johnstone et al. 1998; Schaefer et al. 2002; Tognana et al. 2005; Martin et al. 2007).
Our aim was to investigate if osteochondral tissue composites consisting of a cartilage region and a bone region can be formed using human mesenchymal stem cells (hMSCs), by differential application of osteogenic (β-glycerol phosphate, BMP-2) and chondrogenic (insulin, TGF-β) regulatory factors, the effects of which on hMSCs were established in our previous studies (Meinel et al. 2004a,b). The main focus of work was on the effects of chondrogenic and osteogenic factors on the formation and integration of these composite constructs. We selected the cell source (hMSCs), scaffold type (porous silk) and bioreactor system (rotating vessels) based on our previous studies of tissue-engineered cartilage- and bone-like tissues. In these studies, hMSCs were shown to differentiate into chondrogenic and osteogenic lineages when cultured on silk scaffolds and subjected to chondrogenic or osteogenic regulatory factors (Meinel et al. 2004a–c; Kim et al. 2005a; Wang et al. 2005; Hofmann et al. 2006; Marolt et al. 2006).
The hMSCs were selected for the present work because biosynthetically active hMSCs can be harvested from bone marrow aspirates of most patients and expanded in culture. These cells have the ability to be selectively differentiated into either cartilage or bone lineages. Therefore, hMSCs are considered a suitable and clinically relevant source for engineering of osteochondral grafts. Silk scaffolds were selected owing to their high biocompatibility (Lange 1907; Meinel et al. 2005), slow degradation and the availability of techniques for generating custom-designed scaffold architectures with controlled porosity, pore size and mechanical properties (Altman et al. 2002, 2003; Kim et al. 2005b). Rotating bioreactors were selected owing to their proven ability to support the formation of large, homogenous mineralized bone- and cartilage-like constructs by hMSCs cultured on silk scaffolds (Marolt et al. 2006). The dynamics of the hydrodynamic and mass transport environment in rotating bioreactors interacted synergistically with growth factors (Gooch et al. 2001; Pei et al. 2002) and yielded tissue constructs with compositions and functional properties superior to other culture systems studied, such as spinner flasks and Petri dishes (Freed et al. 1998; Vunjak-Novakovic et al. 1999, 2002; Martin et al. 2000; Pei et al. 2002).
To investigate the differential effects of osteogenic and chondrogenic regulatory factors on the formation of human cartilage–bone tissue composites, we cultured hMSCs on silk scaffolds in rotating bioreactors first individually (to obtain cartilage and bone tissue constructs) and then as cartilage–bone composites, in chondrogenic, osteogenic or control (unsupplemented) media. Our findings emphasize the need for the design of culture systems emulating certain physiological conditions for engineering complex grafts, in order to gain the necessary insights into the combined and sequential effects of regulatory factors and derive protocols that can yield structurally and mechanically functional tissue grafts.
2. Materials and methods
2.1 Materials
Foetal bovine serum (FBS), Dulbecco's modified Eagle's medium (DMEM), basic fibroblast growth factor (bFGF), penicillin–streptomycin (Pen–Strep), Fungizone, Dulbecco's PBS (D-PBS), non-essential amino acids, trypsin/EDTA and Trizol were from Invitrogen (Carlsbad, CA). Ascorbic acid-2-phosphate, insulin, dexamethasone, β-glycerophosphate, calf thymus DNA standard (type I), papainase, Triton X-100 and trypsin were from Sigma-Aldrich (St Louis, MO). Transforming human growth factor-β1 (hTGF-β1) was from R&D Systems (Minneapolis, MN) and gadolinium-diethylenetriamine pentaacetic acid (Gd(DTPA)2−) was from Magnevist (Berlex Imaging, Wayne, NJ). Sodium carbonate and methanol were from Fisher Scientific (Pittsburgh, PA). Silkworm cocoons were kindly supplied by M. Tsukada (Institute of Sericulture, Tsukuba, Japan) and Marion Goldsmith (University of Rhode Island, Cranston, RI). Recombinant human bone morphogenic protein-2 (rhBMP-2) was a gift from Wyeth Biopharmaceuticals (Andover, MA). All other substances were of analytical or pharmaceutical grade and obtained from Sigma-Aldrich.
2.2 Scaffold preparation
Silk scaffolds were prepared from a solution of silk fibroin protein by a slight modification of a previously described porogen-leaching method (Nazarov et al. 2004; Kim et al. 2005b; Vepari & Kaplan 2006). Cocoons from Bombyx mori (Linnaeus 1758) were boiled for 1 hour in an aqueous solution of 0.02 M Na2CO3 and rinsed with water to extract sericins. Purified silk fibroin was solubilized using 9.3 M LiBr solution and dialysed against water for 3 days in a Slide-A-Lyzer of MWCO 3500 (Pierce Chemicals, Rockford, IL). The resulting silk solution was lyophilized and dissolved in hexafluoro-2-propanol (HFIP) at a concentration of 17% (w/v) silk fibroin. Granular NaCl (300–425 μm diameter particles) was used as a porogen. A mixture of NaCl and silk–HFIP solution in a 20 : 1 w/w ratio (corresponding to an approx. 95% volume fraction of NaCl particles) was placed in a closed Teflon container and equilibrated overnight at 4°C. The HFIP was then allowed to evaporate over a period of 3 days, and the resulting blocks were immersed in methanol for 30 min to induce a β-sheet structural transition of the silk fibroin. Scaffolds were dried to remove methanol, and the NaCl particles were then dissolved in water. The resulting porous material (95.5% void volume, pore sizes of 300–425 μm) was kept submerged in water, die-punched into 8 mm diameter×2 mm thick discs using a dermal punch (Miltey, Lake Success, NY) and autoclave sterilized.
2.3 Cell isolation and expansion
Bone marrow-derived hMSCs were isolated, expanded and tested for their differentiation potential as previously described (Meinel et al. 2004a,b; Wang et al. 2006). Briefly, a bone marrow aspirate (50 ml, Cambrex, East Rutherford, NJ) was diluted in expansion medium (DMEM supplemented with 10% FBS, 1 ng ml−1 bFGF, 0.1 mM non-essential amino acids, 1 U ml−1 Pen–Strep, and 2.5 μg ml−1 Fungizone) and plated in tissue culture flasks at a density of 2×105 cells cm−2. Non-adherent cells were removed with subsequent media changes, and the adherent cells (hMSC) were cultured (§ 2.4) to 80% confluence (P0), trypsinized and frozen in a medium consisting of 80% FBS, 10% DMEM, 10% DMSO. Subsequent expansions were started at an initial cell density of 5×103 cells cm−2 and carried out in expansion medium. The second passage (P2) cells were used for FACS analyses, differentiation studies in pellet culture and scaffold seeding.
2.4 Bioreactor cultivation of cartilage- and bone-like constructs
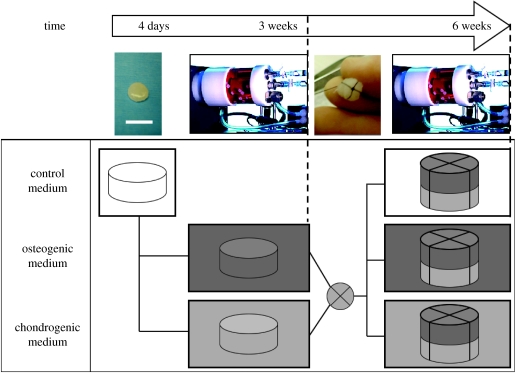
The experimental design is shown in figure 1. Silk scaffolds were incubated overnight in DMEM, blotted dry and placed in 12-well non-tissue culture-treated Petri dishes. Scaffolds were seeded with second passage hMSCs, using 5×106 cells in 100 μl DMEM, and placed in a humidified 37°C incubator. The seeded scaffolds were flipped every 30 min and 10 μl of DMEM was added each time. Under these conditions, the cells remained fully hydrated by the silk sponge that retained culture medium, and by addition of 10 μl of medium that compensated for any evaporation. After 2 hours, 2.5 ml of control media was added to each well and the cell-seeded scaffolds were further incubated for 4 days with complete medium change every day, as in our recent previous study (Marolt et al. 2006). After 4 days, constructs were transferred into rotating bioreactors, using 18 constructs and 110 ml of media per vessel (STLV, Synthecon, Houston, TX).
Figure 1.
Experimental design. Human mesenchymal stem cells (hMSC) were derived from bone marrow aspirates, expanded in culture, seeded into disc-shaped porous silk scaffolds (8 mm diameter×2 mm thick) and cultured statically in control medium without specific chondrogenic or osteogenic supplements for 4 days to promote cell attachment. The resulting constructs were randomly divided into two groups and cultured for three weeks in rotating bioreactors in either osteogenic or chondrogenic medium (please see § 2.4 for the specification of medium supplements). After three weeks, tissue composites were formed by suturing together two constructs; one from the osteogenic and the other from the chondrogenic group. Composites were cultured in bioreactors for an additional three weeks, in osteogenic, chondrogenic or control medium. At three- and six-week time points, the cultured tissues were harvested and evaluated (scale bar, 1 cm).
Three different culture media were established. Control medium consisted of DMEM with 10% FBS, 1% Pen–Strep and 0.2% Fungizone. Chondrogenic medium (CHM) consisted of control medium further supplemented with 50 μg ml−1 ascorbic acid-2-phosphate, 10 nM dexamethasone, 0.1 mM non-essential amino acids, 5 μg ml−1 insulin and 5 ng ml−1 hTGF-β1. Osteogenic medium (OSM) consisted of control medium further supplemented with 50 μg ml−1 ascorbic acid-2-phosphate, 10 nM dexamethasone, 7 mM β-glycerophosphate and 1 μg ml−1 rhBMP-2.
For a period of three weeks, constructs were cultured individually in either osteogenic or chondrogenic medium to engineer bone- or cartilage-like constructs, respectively. Bioreactors were placed into the incubators and operated as previously described. The constructs and culture medium were housed in an annular space between the outer polycarbonate cylinder and the internal silicon membrane providing gas exchange for oxygen and pH control (Freed et al. 1998). Our previous experimental and modelling studies showed that the bioreactor provided efficient exchange of oxygen and carbon dioxide and maintained steady levels of oxygen concentrations and pH in culture medium (Obradovic et al. 1999, 2000). The rate of vessel rotation was set to 16 r.p.m., a value needed to balance the gravity, buoyancy and drag forces at the settling constructs and to maintain the constructs freely suspended around a steady position within the vessel. Culture medium was replaced at a rate of 50% twice per week, using stock media containing 2× concentrations of growth factors, in order to maintain approximately the same initial concentrations of growth factors after each medium change.
2.5 Bioreactor cultivation of tissue composites
After three weeks of individual culture, one construct from the osteogenic group and one construct from the chondrogenic group were randomly selected and joined by a silk suture (5-0 Silk suture, Ethicon) into a composite construct. The two cylindrical constructs (8 mm diameter×2 mm thick) were tied together passing a suture around the composite crosswise to yield parcel-like composites (8 mm diameter×4 mm thick) with the knot positioned at the bone-like phase, and returned to rotating bioreactors (10 composites per vessel in a 110 ml volume). To control the amount of compression exerted through the knot, each knot was tied over the tip of a scalpel holder placed on the stacked constructs, ensuring consistent compression and tightness of suture. A total of 30 composites was formed and randomly divided into three groups of 10 composites. The remaining individual constructs were sampled for analyses of the two regions of the composite (cartilage- and bone-like) at the three-week time point, as described below. Composite constructs were cultured for an additional three weeks in osteogenic, chondrogenic or control medium (figure 1). The rate of bioreactor vessel rotation was measured and set to 19 r.p.m., a rotation rate needed to maintain the composites in a state of dynamic suspension. Culture medium was replaced at a rate of 50% twice per week, using stock media containing 2× concentrations of growth factors, as had been done during the individual construct cultivation.
2.6 Sample allocation
At the three-week time point (end of the individual construct cultivation and the beginning of the composite culture), three of a total of 36 constructs per group (two groups: osteogenic and chondrogenic medium) were used for micro-computerized tomography imaging (μCT) and subsequent histological analyses, and the three constructs per group were cut into quarters and used for biochemical analyses. At the six-week time point (end of composite cultivation), three of the 10 composites per group (three groups: osteogenic, chondrogenic and control medium) were used for magnetic resonance imaging (MRI), followed by μCT and histological analyses. The cartilage-like regions of four composites from each group were used for mechanical testing followed by biochemical assays to determine the DNA and glycosaminoglycans (GAG) contents. Three composites per group were separated into the bone- and cartilage-like layers and used for measurement of alkaline phosphatase (AP) activity and calcium (Ca2+) content. All biochemical parameters were normalized to sample wet weight (ww).
2.7 Imaging analyses (MRI and μCT)
In MRI studies, the gadolinium-enhanced MRI of cartilage (dGEMRIC) method was used to visualize GAG (Bashir et al. 1999). The constructs were equilibrated overnight in saline or saline containing 1 mM of the charged MRI contrast agent Gd(DTPA)2−. The T1 measurements were done on a 8.45 T Bruker magnetic resonance micro-imaging system (Bruker Instruments, Billerica, MA). Images were obtained with a field of view of 25.6 mm and a matrix of 256×256, yielding a pixel resolution of 0.1 mm. For calculation purposes, T1 was measured twice for each construct, first after equilibration with saline to find the MRI parameter T10, and again after equilibration with saline containing Gd(DTPA)2−. A saturation recovery pulse sequence was used to measure T10 with 10 delays ranging from 0.1 to 5 seconds and from 0.1 to 2.7 s for T1Gd. Tissue Gd(DTPA)2− concentration was calculated from T1Gd and T10. Fixed charge density was obtained from the calculated Gd(DTPA)2− concentration via a modified Donnan equilibrium theory from which GAG can in turn be estimated as previously described (Bashir et al. 1999).
In μCT studies of bone-like constructs and cartilage–bone-like composites, the constructs were washed in D-PBS, fixed in 10% neutral-buffered formalin (24 hours, room temperature) and scanned using a μCT imaging system (μCT-40, Scanco Medical, Bassersdorf, Switzerland). The constructs were immersed in saline to homogenize the background in the image and provide better contrast with the mineralized scaffold, and then scanned with a 70 kV peak X-ray energy, 300 ms integration time, 1024 pixel matrix, and 0.016 mm cubic voxel side length. A constrained Gaussian filter was used to suppress noise. All samples were analysed using the same filter width (0.7). Mineralized tissue was segmented from non-mineralized tissue using a threshold that best matched the scaffold structure in a binary image to its structure in the grey scale image. Total volume of the sample, mineralized tissue volume, mineralized tissue volume fraction and trabecular thickness were measured in each sample three dimensionally, without any assumptions about the object's geometry (Parfitt et al. 1983, 1987; Hildebrand & Rüegsegger 1997; Hildebrand et al. 1999).
Constructs evaluated by both imaging methods (all composites and some individual constructs serving as negative controls) were first subjected to MRI, washed in D-PBS and then prepared for μCT as described above.
2.8 Histology and immunostaining
Imaged constructs were subsequently dehydrated in graded ethanol solutions, embedded in paraffin, bisected through the centre and cut into 5 μm thick sections. Histological staining for the presence of GAG (alcian blue) and matrix mineralization (von Kossa) was done as previously described (Meinel et al. 2004b; Wang et al. 2005). Immunohistochemical staining for collagen types I and II was done using established methods from our previous studies (Marolt et al. 2006). The histological sections were deparaffinized, sequentially treated with trypsin (1 mg ml−1, 15 min at 37°C), H2O2 (0.03%, 30 min at room temperature) and normal horse serum (10% in PBS, 30 min at room temperature) and incubated overnight (4°C in a humidified chamber) with monoclonal antibodies against human collagen type I (diluted 1 : 100) and collagen type II (diluted 1 : 150) both obtained from US Biological (Swampscott, MA, USA). Secondary antibody was applied after washing, developed with chromagen DAB solution (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA) and the sections were counterstained with haematoxylin.
2.9 Mechanical testing
Compressive moduli of the cartilage-like regions of each composite were assessed using an ElectroForce test system (ELF 3200, Bose Corporation, Framingham, MA) as previously described (Tognana et al. 2005). In brief, composites were separated and 6 mm diameter samples were die-punched, equilibrated in PBS for 10 min, and placed in a chamber custom designed to provide radial confinement while permitting axial fluid flow via a stainless steel filter with 50 μm pores. The chamber was filled with PBS and connected to the ELF 3200 fitted with a 250 g load cell. Five consecutive stress relaxations were applied, each consisting of a 3% strain step followed by a 600 s dwell. The constructs were considered to relax fully during this increment based on a change in stress of less than 0.003 MPa over the final 180 s. Data were recorded using a 10 Hz filter at an average sampling rate of 2 points s−1. Equilibrium modulus was determined from the slope of the best linear regression fit (R2>0.97) of equilibrium stress versus applied strain.
2.10 Biochemical analyses
Following mechanical testing, the 6-mm discs and the remaining construct material were carefully collected, frozen, weighed, lyophilized, reweighed and digested (0.14 mg ml−1 papainase in 100 mM phosphate buffer with 10 mM EDTA and 10 mM cysteine) for 20 hours at 60°C, using 2 ml of the enzyme solution per sample (Freed et al. 1998).
DNA content was determined fluorometrically following the binding of Hoechst 33258 dye (Polysciences, Warrington, PA) using DNA from calf thymus (Sigma D-1501) as a standard (Kim et al. 1988). The GAG content was determined spectrophotometrically at 656 nm following the reaction with dimethylmethylene blue dye, according to the manufacturer's protocol (Blyscan, Biocolor Ltd., Newtownabbey, Northern Ireland).
2.10.1 Ca2+ assay and AP activity
To measure the calcium content, samples were extracted twice with 5% trichloroacetic acid (1 and 0.5 ml per sample) using steel balls and a Minibeadbeater (Biospec, Bartlesville, OK; Meinel et al. 2004c,a). Calcium content was determined spectrophotometrically at 550 nm, following the reaction with o-cresolphthalein complexone according to the manufacturer's protocol (Calcium (CPC) LiquiColor Test, Stanbio Laboratory, Boerne, TX). For measurement of AP activity, samples were extracted (as described above) with 5 mM MgCl2/0.2% Triton X-100 solution (1 ml per sample) and the activity was measured using a biochemical assay from Sigma-Aldrich, based on conversion of p-nitrophenyl phosphate to p-nitrophenol, which was measured spectrophotometrically at 405 nm.
2.11 Statistical analyses
Statistical significance was determined by one-way ANOVA with Scheffe's post hoc test or multivariate ANOVA with the Tukey HSD test using Statistica v. 7 (StatSoft, Inc., Tulsa, OK). Differences between groups with p<0.05 were considered statistically significant.
3. Results
3.1 Expression of cartilage and bone markers
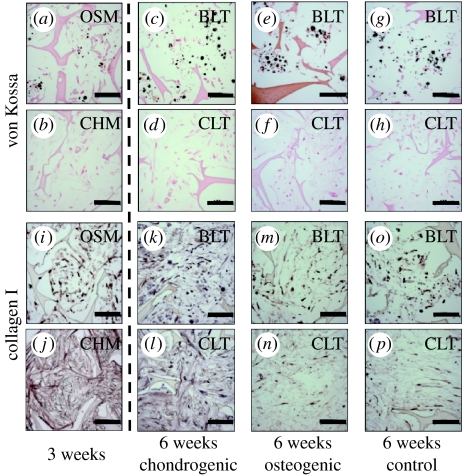
After three weeks of individual culture, calcium deposition evidenced by von Kossa stain was observed in constructs cultured in OSM and not in those cultured in chondrogenic medium (figure 2a,b). After an additional three weeks of culture (six weeks of total culture), calcium deposition was again observed only in constructs cultured in OSM during the first three weeks, irrespective of culture medium used during weeks 3–6 (figure 2c,e,g). Notably, the cartilage-like region contained no mineralized regions even in the OSM group (figure 2d,f,h). Type I collagen was found in constructs from all groups, at both time points (three and six weeks; figure 2i–p).
Figure 2.
Histomorphologies of cartilage- and bone-like regions. (a,b) Representative histological sections are shown for tissue constructs cultured individually for the first three weeks in OSM or CHM, and (c–h,k–p) for the cartilage-like (CLT) and bone-like (BLT) regions of composites cultured for an additional three weeks in chondrogenic, osteogenic or control medium. Stains: (a–h) von Kossa and (i–p) collagen type I immunostain (scale bars, 100 μm).
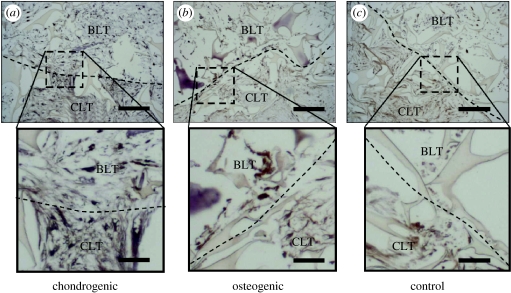
Chondrogenic medium resulted in better integration of the cartilage- and bone-like regions in tissue composites, as observed during attempted separation of the parts. Composites cultured in CHM had to be cut apart using a microtome blade, whereas composites in osteogenic and control media could be separated with minimal force, i.e. by gently pulling the two phases apart using two pairs of forceps. Consistent with this observation, the interfacial region of composites cultured in CHM exhibited a network of collagen spanning between the cartilage- and bone-like regions (figure 3a), whereas in osteogenic and control media collagen was less prevalent at the interface (figure 3b,c). The interface could easily be identified in all groups, and the composites could be reproducibly separated into cartilage and bone regions. In all groups, constructs harvested after three and six weeks of culture exhibited only minimal staining for GAG and type II collagen.
Figure 3.
Histomorphologies of composite constructs. Representative histological sections of the interface regions between cartilage-like (CLT) and bone-like (BLT) regions are shown for six-week constructs cultured in (a) chondrogenic, (b) osteogenic or (c) control medium stained with collagen type I antibody. Dotted lines indicate the separation between the two phases (scale bars, 200 μm (inserts: 50 μm)).
3.2 Biochemical compositions of bone- and cartilage-like regions
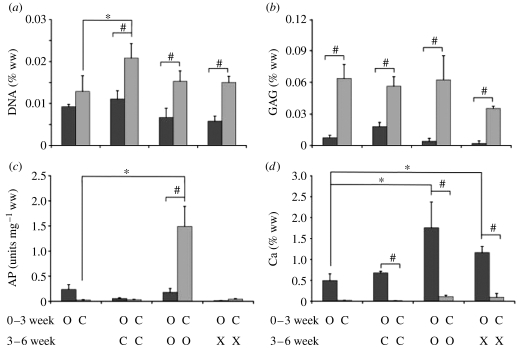
Effects of culture time and medium composition on individual three-week constructs and the cartilage- and bone-like regions of six-week composites were assessed in terms of DNA, GAG, calcium and AP contents, as a function of medium supplements—chondrogenic, osteogenic or none—during the culture (figure 4).
Figure 4.
Biochemical compositions of cartilage- and bone-like regions. Biochemical analyses are shown for the individually cultured cartilage- and bone-like constructs (three-week time point), and for the bone- and cartilage-like regions of six-week composites cultured in chondrogenic (C), osteogenic (O) or control medium (X). (a) DNA concentration (% tissue ww), (b) GAG concentration (% tissue ww; c), AP activity (units mg−1 tissue ww) and (d) calcium concentration (% tissue ww). Dark grey bars correspond to bone-like regions, and light grey bars correspond to cartilage-like regions. Data represent mean±s.d. (n=4 samples per group and time point). Statistically significant differences between the three-week constructs and the cartilage- or bone-like region of the six-week composites are denoted by ‘*’. Statistically significant difference between the groups at the six-week time point are denoted by ‘#’ (p<0.05).
DNA content (% ww) was higher for the cartilage-like region than for the bone-like region in all groups, at both time points. During weeks 3–6 of culture, the DNA contents increased significantly only in the cartilage-like regions of composites cultured in CHM. In addition, the DNA contents in the composites cultured in CHM was slightly but not significantly higher for both the cartilage- and bone-like regions when compared with the cartilage- and bone-like regions in either the osteogenic or control medium group (figure 4a).
The GAG content (% ww) in three-week constructs and six-week composites was significantly higher in the cartilage-like regions than in the bone-like regions. The amounts of GAG in the cartilage- and bone-like regions remained relatively constant over the time of culture in all groups, except for the composites cultured in chondrogenic (increase in GAG content in the bone-like region) and control mediums (decrease in GAG content in the cartilage-like region; figure 4b).
The construct AP activity (U mg−1 ww) was rather high in the cartilage-like region of composites cultured in OSM (figure 4c). In all other groups, the AP activity was significantly lower and comparable, at both time points, with the AP activity measured in our previous study (Marolt et al. 2006).
The AP activity (U mg−1 ww) of the bone-like constructs after three weeks of culture was approximately 30% lower than previously measured for five-week constructs (approx. 0.4 U mg−1 ww; Marolt et al. 2006). After an additional three weeks of composite culture in OSM, the AP activity remained relatively constant in the bone-like region and markedly increased in the cartilage-like region (figure 4c).
In all groups and time points, the Ca2+ content (% ww) was relatively high in the bone-like regions of constructs and composites, and below detectable levels in cartilage-like regions. The Ca2+ content increased in a statistically significant manner with time of culture in bone-like regions of composites cultured in osteogenic and control media, to reach the highest levels after six weeks of culture for the OSM group (figure 4d).
3.3 Imaging and mechanical characterization of tissue composites
Analysis of six-week composites using MRI showed visible distinction between the cartilage- and bone-like regions in all three groups (figure 5a). Owing to very low GAG levels in constructs at three as well as six weeks, it was not possible to calculate maps of T1 relaxation and hence GAG deposition.
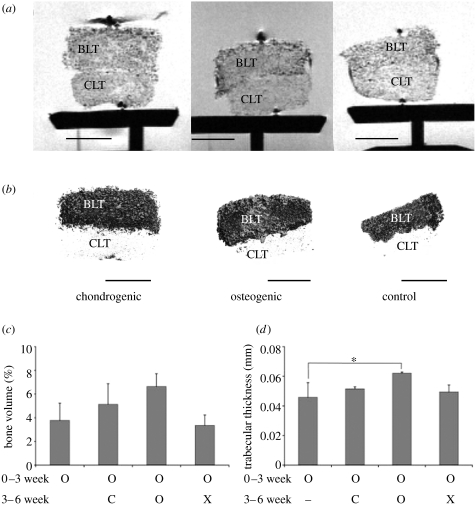
Figure 5.
Structural properties of six-week tissue composites. Imaging assessments are shown for the cartilage-like (CLT) and bone-like (BLT) regions of six-week composites cultured in chondrogenic (C), osteogenic (O) or control medium (X). (a) MRI density maps, (b) volume-rendered μCT images, (c) volume fractions of mineralized tissue determined from μCT images, and (d) trabecular thicknesses measured in the mineralized regions from μCT images. Data represent mean±s.d. (n=4 per data point; scale bars, 4 mm).
Analysis of six-week composites by μCT showed mineralization in the bone regions of composites from all three groups (figure 5b). The volume of newly formed bone in the bone regions of composites cultured in OSM was approximately 7%, the highest among all groups (figure 5c). Bone trabecular thickness measured approximately 60 μm for composites cultured for six weeks in OSM, a value higher than for the composites from the other two groups, and the three-week constructs cultured in OSM (figure 5d).
Compressive moduli of constructs cultured individually for three weeks in CHM and then as composites for an additional three weeks in chondrogenic, osteogenic or control media were 21±5.1, 18±3.6 and 31±7.4 kPa, respectively, with no statistically significant differences between the three groups. For comparison, the compressive modulus of empty scaffolds cultured under the same conditions and assessed in our previous study was 7.7±2.2 kPa (Marolt et al. 2006).
4. Discussion
We explored the effects of osteogenic and chondrogenic regulatory factors on the formation and integration of the composite constructs grown using hMSCs, silk scaffolds and rotating bioreactors. Our aim was to get more insight into the capacity of hMSCs for selective differentiation into osteochondral tissues, when cultured on three-dimensional scaffolds first individually and then as composites, in osteogenic, chondrogenic or control media. This model enabled us to study and compare the responses of the same population of hMSCs to chondrogenic and osteogenic regulatory factors, and to determine how these responses were modulated by the presence of already differentiated hMSCs in the adjacent tissue construct within the cartilage–bone-like composite. The results that we report have clear implications for our understanding of hMSC commitment and maintenance of their phenotype, in the context of the in vitro formation of osteochondral grafts.
In previous studies, bone-like (Meinel et al. 2004c) and cartilage-like (Mauck et al. 2003; Meinel et al. 2004a; Marolt et al. 2006) tissue constructs were generated separately starting from hMSCs. These studies provided evidence for selective chondrogenic and osteogenic differentiation of hMSCs in response to the same combinations of regulatory factors, and within a similar time frame as in the present study. In other studies, cartilage- and bone-like components were combined into osteochondral composites, using primary bovine chondrocytes and bovine periosteal cells (Schaefer et al. 2000), human foetal chondrocytes and human foetal osteoblasts (Mahmoudifar & Doran 2005), or human articular chondrocytes and hMSCs (Pound et al. 2007). A highly relevant study included the engineering of osteochondral composites by press fitting a pellet of chondrogenic cells onto the porous polymer scaffold loaded with osteogenic cells derived from trabecular bone (Tuli et al. 2004). After 10 weeks of cultivation, a layer resembling hyaline cartilage formed over the bone-like phase, with an interface resembling the native osteochondral junction. Our approach used the hMSCs derived from bone marrow aspirates for deriving both the chondrogenic and osteogenic cell populations, and showed some important differences in the capacity of hMSCs for osteogenic and chondrogenic differentiation, and their responses to chondrogenic and osteogenic factors.
As expected, calcium deposition at three weeks was observed only in constructs cultured in OSM and not in constructs cultured in CHM (figure 2a,b). Interestingly, calcium deposition in these constructs persisted during subsequent culture of up to six weeks, in all three culture media, as evidenced by von Kossa staining (figure 2c,e,g), biochemically measured calcium content (figure 4d) and μCT imaging (figure 5b). In contrast, when constructs initially cultured in chondrogenic medium were subjected to OSM, mineral deposition could not be observed (figure 2d,f,h). These results suggest that osteogenic factors applied early during culture (less than or equal to three weeks) were necessary to induce mineralization, and that this effect could not be induced by application of osteogenic factors later during culture. Further studies are needed to test the mechanisms underlying this lack of sensitivity to osteogenic factors after a period of exposure to non-osteogenic factors.
During subsequent composite culture, the amount of calcium markedly and significantly increased in the bone-like region in both the osteogenic and control medium groups, with the highest amounts of calcium deposition observed in the group constantly subjected to OSM (during three weeks of individual culture, and the additional three weeks of composite culture; figure 4d). The μCT analyses showed the corresponding increases in bone-like volume and trabecular bone thickness (figure 5c,d). Taken together, these data suggest that there is a robust response of hMSCs to the early application of osteogenic factors that persists during subsequent cultivation, with evidence of bone-like formation in composites from all three groups. The maximum volume fraction of mineralized bone of approximately 7% (figure 5c) is approaching the range of values measured for native bone (Legrand et al. 2000).
In contrast, the progression of chondrogenesis was slower and much less complete, as evidenced by relatively low concentrations of GAG and undetectable levels of type II collagen. Notably, the progression of chondrogenesis was also slow relatively to chondrogenesis in pellet cultures of hMSCs (Marolt et al. 2006). When GAG content measured in the present study was normalized to DNA content, the ratio in the chondrogenic group (2.9 g g−1) was the same as in two previous studies of hMSC chondrogenesis on silk scaffolds (2.9 μg μg−1 in Marolt et al. 2006, 3.2 μg μg−1 in Meinel et al. 2004a). Taken together, these data from three independent studies that used hMSCs and silk scaffolds show that the overall DNA and GAG contents remain markedly lower than in corresponding constructs grown using bovine calf MSCs (Martin et al. 2001) or bovine calf articular chondrocytes (Freed et al. 1998; Vunjak-Novakovic et al. 1999; Pei et al. 2002).
Compressive properties of constructs cultured in chondrogenic media, first individually and then as composites developed with time in culture, and the measured compressive moduli (20.8±5.1 kPa) were similar to those we previously measured for hMSC-based constructs cultured in chondrogenic medium (26.1±8.9 kPa; Marolt et al. 2006), and higher than those we previously measured for silk scaffolds without cultured cells (7.7±2.2 kPa; Marolt et al. 2006). The GAG deposition in constructs cultured for three weeks in chondrogenic medium was low and did not increase with time during subsequent cultivation of composites in any type of medium (figure 4a). Lack of significant GAG deposition impaired quantitative assessment by dGEMRIC, even though it was possible to clearly distinguish the two tissue types on the T1 maps (figure 5a).
The difficulties in forming cartilaginous extracellular matrix (ECM) may be due to the scaffold properties, i.e. the large pore size and high porosity, which may cause washout of GAG molecules before they are immobilized in tissue matrix. In order to test this hypothesis, studies should be done to separately determine GAG synthesis and GAG deposition using radiolabelling techniques. It has recently been shown that alginate hydrogels within PGA scaffolds can aid GAG and collagen type II formation, probably due to retention of ECM molecules and chondrogenic growth factors within the construct (Hannouche et al. 2007) or the maintenance of the appropriate cell shape, found in native cartilage.
Other studies indicate that the reasons for poor chondrogenesis in hMSCs are more fundamental. Recent systematic studies showed that the GAG contents and mechanical properties were significantly lower for cartilage-like constructs based on bovine MSCs than for those based on bovine chondrocytes, and did not improve with time in culture (Mauck et al. 2006). Importantly, these differences were not due to a delayed chondrogenesis in MSCs, but rather to some other intrinsic differences between the two cell types. Our study supports the notion that the formation of hMSC-based functional engineered cartilage will require further insights into the regulation of chondrogenesis in these cells and elucidate the influence of other factors such as the presence of serum in the culture medium or hydrostatic pressure.
Chondrogenic conditions were associated with enhanced cell proliferation. DNA increased with time for constructs initially cultured in chondrogenic medium and was the highest for the cartilage-like regions of composites cultured in chondrogenic medium (figure 4a). These data indicate that one of the supplemental chondrogenic factors (e.g. insulin or TGF-β) enhanced hMSC proliferation over chondrogenic differentiation. In the bone regions of composites, the slight but not statistically significant decrease in the ww fraction of DNA is most likely due to the deposition of mineralized tissue. We reported a similar observation in our previous study with the same experimental system for cartilage- and bone-like constructs cultured for five weeks (Marolt et al. 2006), while other reports (Martin et al. 2001) indicate that TGF-β stimulated proliferation in other culture systems. Alternatively, high proliferation may be associated with hypertrophy, a cell behaviour previously reported for chondrogenic differentiation of MSCs (Winter et al. 2003).
Chondrogenic conditions applied early in culture were also associated with high activity of AP in cartilage-like regions of constructs cultured as composites in OSM (figure 4c). These constructs showed no mineralization, as evidenced by three different methods (figures 2, 4d and 5b). The AP and osteopontin gene expression were previously reported for chondrogenic micromass culture of hMSCs (Pittenger et al. 1999) albeit at low levels. Because AP is considered a marker for early stages of osteogenic differentiation (Stucki et al. 2001), we cannot exclude the possibility of cell reprogramming in response to osteogenic factors as shown previously (Muraglia et al. 1998).
Chondrogenic regulatory factors applied during composite cultivation enhanced the integration of composite constructs (figure 3a). This important effect may be associated with increased collagen deposition (figure 3a) and cell proliferation (figure 4a) in the cartilage-like region of the composites cultured in chondrogenic medium when compared with the osteogenic and control medium. These findings seem consistent with previous studies of the integration between engineered cartilage and either native cartilage or bone (Obradovic et al. 2001; Mahmoudifar & Doran 2005). In all these studies, the presence of biosynthetically active cells was required for the synthesis and deposition of new tissue matrix forming a bond at the tissue interface. The collagen network spanning between the two regions was observed only when composites were cultured in chondrogenic medium. Therefore, chondrogenic factors play a major role in the engineering and integration of osteochondral tissues.
5. Conclusion
The study shows that it is possible to engineer osteochondral composites using hMSCs derived from bone marrow, porous silk scaffolds and bioreactors. Results suggested that in this system the culture medium composition is of greater importance than the co-culture of two different cell types. While the generation of bone-like material was successful, generation of cartilage-like material proved more difficult. Osteogenic factors (β-glycerol phosphate, BMP-2) initiated mineralization in the bone-like region early during culture. The observed development and maintenance of the mineralized bone-like tissue in the presence of both osteogenic and chondrogenic factors was considered as evidence of robust osteogenesis and stable osteogenic phenotype. Chondrogenic factors (insulin, TGF-β) enhanced integration of the cartilage- and bone-like regions in tissue composites, which is attributed to enhanced cell proliferation and the formation of a network of collagen at the cartilage–bone interface. The present study paves the way for developing better strategies for engineering osteochondral tissue composites with the cartilage- and bone-like regions generated from the same initial population of hMSCs. Selective differentiation of adult hMSCs into functional tissue grafts with clinical use will require additional work before the instructive interactions between cells from the two regions can be fully used.
Acknowledgments
We would like to thank William Yongzhong Wang for help with hMSC characterization, Hyeon Joo Kim for help with silk scaffolds and Hyoungshin Park for help with histology. We would also like to thank Thomas Porter from Wyeth Biopharmaceuticals for the generous gift of rhBMP-2. This work was supported by the National Institutes of Health (P41 EB002520-01A1, RO1 DE016525-01), the National Aeronautics and Space Administration (NNJ04HC72G) and the Ministry of Higher Education, Science and Technology of the Republic of Slovenia (L4-6325-0311-04/4.06, 3311-01-831/476).
References
- Alhadlaq A, et al. Adult stem cell driven genesis of human-shaped articular condyle. Ann. Biomed. Eng. 2004;32:911–923. doi: 10.1023/B:ABME.0000032454.53116.ee. [DOI] [PubMed] [Google Scholar]
- Altman G.H, Horan R.L, Lu H.H, Moreau J, Martin I, Richmond J.C, Kaplan D.L. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23:4131–4141. doi: 10.1016/S0142-9612(02)00156-4. [DOI] [PubMed] [Google Scholar]
- Altman G.H, Diaz F, Jakuba C, Calabro T, Horan R.L, Chen J, Lu H, Richmond J, Kaplan D.L. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/S0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- Bartha L, Vajda A, Duska Z, Rahmeh H, Hangody L. Autologous osteochondral mosaicplasty grafting. J. Orthop. Sports Phys. Ther. 2006;36:739–750. doi: 10.2519/jospt.2006.2182. [DOI] [PubMed] [Google Scholar]
- Bashir A, Gray M.L, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn. Reson. Med. 1999;41:857–865. doi: 10.1002/(SICI)1522-2594(199905)41:5%3C857::AID-MRM1%3E3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Freed L.E, Hollander A.P, Martin I, Barry J.R, Langer R, Vunjak-Novakovic G. Chondrogenesis in a cell-polymer-bioreactor system. Exp. Cell Res. 1998;240:58–65. doi: 10.1006/excr.1998.4010. [DOI] [PubMed] [Google Scholar]
- Gooch K.J, Kwon J.H, Blunk T, Langer R, Freed L.E, Vunjak-Novakovic G. Effects of mixing intensity on tissue-engineered cartilage. Biotechnol. Bioeng. 2001;72:402–407. doi: 10.1002/1097-0290(20000220)72:4%3C402::AID-BIT1002%3E3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Hannouche D, Terai H, Fuchs J.R, Terada S, Zand S, Nasseri B.A, Petite H, Sedel L, Vacanti J.P. Engineering of implantable cartilaginous structures from bone marrow-derived mesenchymal stem cells. Tissue Eng. 2007;13:87–99. doi: 10.1089/ten.2006.0067. [DOI] [PubMed] [Google Scholar]
- Hildebrand T, Rüegsegger P. A new method for the model-independent assessment of thickness in three-dimensional images. J. Microsc. 1997;185:67–75. doi: 10.1046/j.1365-2818.1997.1340694.x. [DOI] [Google Scholar]
- Hildebrand T, Laib A, Muller R, Dequeker J, Ruegsegger P. Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J. Bone Miner. Res. 1999;14:1167–1174. doi: 10.1359/jbmr.1999.14.7.1167. [DOI] [PubMed] [Google Scholar]
- Hofmann S, Knecht S, Langer R, Kaplan D.L, Vunjak-Novakovic G, Merkle H.P, Meinel L. Cartilage-like tissue engineering using silk scaffolds and mesenchymal stem cells. Tissue Eng. 2006;12:2729–2738. doi: 10.1089/ten.2006.12.2729. [DOI] [PubMed] [Google Scholar]
- Jiang J, Nicoll S.B, Lu H.H. Co-culture of osteoblasts and chondrocytes modulates cellular differentiation in vitro. Biochem. Biophys. Res. Commun. 2005;338:762–770. doi: 10.1016/j.bbrc.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Johnstone B, Hering T.M, Caplan A.I, Goldberg V.M, Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- Kim Y.J, Sah R.L, Doong J.Y, Grodzinsky A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal. Biochem. 1988;174:168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- Kim H.J, Kim U.J, Vunjak-Novakovic G, Min B.H, Kaplan D.L. Influence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials. 2005a;26:4442–4452. doi: 10.1016/j.biomaterials.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Kim U.J, Park J, Kim H.J, Wada M, Kaplan D.L. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005b;26:2775–2785. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Lange F. Künstliche Bänder aus Seide. Münch. Med. Wochenschr. 1907;17:834–836. [Google Scholar]
- Legrand E, Chappard D, Pascaretti C, Duquenne M, Krebs S, Rohmer V, Basle M.F, Audran M. Trabecular bone microarchitecture, bone mineral density, and vertebral fractures in male osteoporosis. J. Bone Miner. Res. 2000;15:13–19. doi: 10.1359/jbmr.2000.15.1.13. [DOI] [PubMed] [Google Scholar]
- Linnaeus, C. 1758 Systema naturae per regna tria naturae, secundum classes ordines, genera, species, differentiis, synonymis, locis, 10th edn. Laurentii Salvii: Holmiae. See http://resolver.sub.uni-goettingen.de/purl?PPN362052875.
- Mahmoudifar N, Doran P.M. Tissue engineering of human cartilage and osteochondral composites using recirculation bioreactors. Biomaterials. 2005;26:7012–7024. doi: 10.1016/j.biomaterials.2005.04.062. [DOI] [PubMed] [Google Scholar]
- Marolt D, et al. Bone and cartilage tissue constructs grown using human bone marrow stromal cells, silk scaffolds and rotating bioreactors. Biomaterials. 2006;27:6138–6149. doi: 10.1016/j.biomaterials.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Martin I, Obradovic B, Treppo S, Grodzinsky A.J, Langer R, Freed L.E, Vunjak-Novakovic G. Modulation of the mechanical properties of tissue engineered cartilage. Biorheology. 2000;37:141–147. [PubMed] [Google Scholar]
- Martin I, Shastri V.P, Padera R.F, Yang J, Mackay A.J, Langer R, Vunjak-Novakovic G, Freed L.E. Selective differentiation of mammalian bone marrow stromal cells cultured on three-dimensional polymer foams. J. Biomed. Mater. Res. 2001;55:229–235. doi: 10.1002/1097-4636(200105)55:2%3C229::AID-JBM1009%3E3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Martin I, Miot S, Barbero A, Jakob M, Wendt D. Osteochondral tissue engineering. J. Biomech. 2007;40:750–765. doi: 10.1016/j.jbiomech.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Mauck R.L, Nicoll S.B, Seyhan S.L, Ateshian G.A, Hung C.T. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- Mauck R.L, Yuan X, Tuan R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthr. Cartil. 2006;14:179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Meinel L, Hofmann S, Karageorgiou V, Zichner L, Langer R, Kaplan D, Vunjak-Novakovic G. Engineering cartilage-like tissue using human mesenchymal stem cells and silk protein scaffolds. Biotechnol. Bioeng. 2004a;88:379–391. doi: 10.1002/bit.20252. [DOI] [PubMed] [Google Scholar]
- Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L, Kaplan D, Langer R, Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann. Biomed. Eng. 2004b;32:112–122. doi: 10.1023/B:ABME.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- Meinel L, et al. Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. J. Biomed. Mater. Res. A. 2004c;71:25–34. doi: 10.1002/jbm.a.30117. [DOI] [PubMed] [Google Scholar]
- Meinel L, Fajardo R, Hofmann S, Langer R, Chen J, Snyder B, Vunjak-Novakovic G, Kaplan D. Silk implants for the healing of critical size bone defects. Bone. 2005;37:688–698. doi: 10.1016/j.bone.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Meinel L, Hofmann S, Betz O, Fajardo R, Merkle H.P, Langer R, Evans C.H, Vunjak-Novakovic G, Kaplan D.L. Osteogenesis by human mesenchymal stem cells cultured on silk biomaterials: comparison of adenovirus mediated gene transfer and protein delivery of BMP-2. Biomaterials. 2006;27:4993–5002. doi: 10.1016/j.biomaterials.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Muraglia A, Martin I, Cancedda R, Quarto R. A nude mouse model for human bone formation in unloaded conditions. Bone. 1998;22:131S–134S. doi: 10.1016/S8756-3282(98)00009-X. [DOI] [PubMed] [Google Scholar]
- Nazarov R, Jin H.J, Kaplan D.L. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5:718–726. doi: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]
- Obradovic B, Carrier R.L, Vunjak-Novakovic G.V, Freed L.E. Oxygen is essential for bioreactor cultivation of tissue engineered cartilage. Biotechnol. Bioeng. 1999;63:197–205. doi: 10.1002/(SICI)1097-0290(19990420)63:2%3C197::AID-BIT8%3E3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Obradovic B, Meldon J.H, Freed L.E, Vunjak-Novakovic G. Glycosaminoglycan deposition in tissue engineered cartilage: experiments and mathematical model. J. Am. Inst. Chem. Eng. (AIChE) 2000;46:1860–1871. [Google Scholar]
- Obradovic B, Martin I, Padera R.F, Treppo S, Freed L.E, Vunjak-Novakovic G. Integration of engineered cartilage. J. Orthop. Res. 2001;19:1089–1097. doi: 10.1016/S0736-0266(01)00030-4. [DOI] [PubMed] [Google Scholar]
- Parfitt A.M, Mathews C.H, Villanueva A.R, Kleerekoper M, Frame B, Rao D.S. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J. Clin. Invest. 1983;72:1396–1409. doi: 10.1172/JCI111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt A.M, Drezner M.K, Glorieux F.H, Kanis J.A, Malluche H, Meunier P.J, Ott S.M, Recker R.R. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Pei M, Solchaga L.A, Seidel J, Zeng L, Vunjak-Novakovic G, Caplan A.I, Freed L.E. Bioreactors mediate the effectiveness of tissue engineering scaffolds. FASEB J. 2002;16:1691–1694. doi: 10.1096/fj.02-0083fje. [DOI] [PubMed] [Google Scholar]
- Pittenger M.F, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Pound J.C, Green D.W, Roach H.I, Mann S, Oreffo R.O. An ex vivo model for chondrogenesis and osteogenesis. Biomaterials. 2007;28:2839–2849. doi: 10.1016/j.biomaterials.2007.02.029. [DOI] [PubMed] [Google Scholar]
- Schaefer D, Martin I, Shastri P, Padera R.F, Langer R, Freed L.E, Vunjak-Novakovic G. In vitro generation of osteochondral composites. Biomaterials. 2000;21:2599–2606. doi: 10.1016/S0142-9612(00)00127-7. [DOI] [PubMed] [Google Scholar]
- Schaefer D, Martin I, Jundt G, Seidel J, Heberer M, Grodzinsky A, Bergin I, Vunjak-Novakovic G, Freed L.E. Tissue-engineered composites for the repair of large osteochondral defects. Arthritis Rheum. 2002;46:2524–2534. doi: 10.1002/art.10493. [DOI] [PubMed] [Google Scholar]
- Sherwood J.K, Riley S.L, Palazzolo R, Brown S.C, Monkhouse D.C, Coates M, Griffith L.G, Landeen L.K, Ratcliffe A. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials. 2002;23:4739–4751. doi: 10.1016/S0142-9612(02)00223-5. [DOI] [PubMed] [Google Scholar]
- Simon T.M, Jackson D.W. Articular cartilage: injury pathways and treatment options. Sports Med. Arthrosc. 2006;14:146–154. doi: 10.1097/00132585-200609000-00006. [DOI] [PubMed] [Google Scholar]
- Stucki U, Schmid J, Hammerle C.F, Lang N.P. Temporal and local appearance of alkaline phosphatase activity in early stages of guided bone regeneration. A descriptive histochemical study in humans. Clin. Oral Implants Res. 2001;12:121–127. doi: 10.1034/j.1600-0501.2001.012002121.x. [DOI] [PubMed] [Google Scholar]
- Tognana E, Chen F, Padera R.F, Leddy H.A, Christensen S.E, Guilak F, Vunjak-Novakovic G, Freed L.E. Adjacent tissues (cartilage, bone) affect the functional integration of engineered calf cartilage in vitro. Osteoarthr. Cartil. 2005;13:129–138. doi: 10.1016/j.joca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Tuli R, et al. Human mesenchymal progenitor cell-based tissue engineering of a single-unit osteochondral construct. Tissue Eng. 2004;10:1169–1179. doi: 10.1089/ten.2004.10.1169. [DOI] [PubMed] [Google Scholar]
- Vepari C.P, Kaplan D.L. Covalently immobilized enzyme gradients within three-dimensional porous scaffolds. Biotechnol. Bioeng. 2006;93:1130–1137. doi: 10.1002/bit.20833. [DOI] [PubMed] [Google Scholar]
- Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky A.J, Langer R, Freed L.E. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J. Orthop. Res. 1999;17:130–138. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- Vunjak-Novakovic G, Obradovic B, Martin I, Freed L.E. Bioreactor studies of native and tissue engineered cartilage. Biorheology. 2002;39:259–268. [PubMed] [Google Scholar]
- Wang Y, Kim U.J, Blasioli D.J, Kim H.J, Kaplan D.L. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials. 2005;26:7082–7094. doi: 10.1016/j.biomaterials.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kim H.J, Vunjak-Novakovic G, Kaplan D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials. 2006;27:6064–6082. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber R.M, Ewerbeck V, Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418–429. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]