Abstract
The ability to adapt and respond to nutrients is an ancient cellular function, conserved from unicellular to the most complex multicellular organisms, including mammals. Mammals adapt to changes in nutritional status through the modulation of tissue-specific metabolic pathways so as to maintain energy homeostasis. At least two proteins are activated in response to reduced nutrient availability: AMP-activated protein kinase (AMPK) and NAD+-dependent deacetylase SIRT1. AMPK functions as a sensor of cellular energy status and as a master regulator of metabolism. When ATP levels decrease, AMPK is activated to boost ATP production and to inhibit ATP usage, thus restoring energy balance. Similarly, SIRT1 is activated in response to changes in the energy status to promote transcription of genes that mediate the metabolic response to stress, starvation, or calorie restriction. Several observations support a model where, in response to stress and reduced nutrients, a metabolic pathway is activated within which AMPK and SIRT1 concordantly function to ensure an appropriate cellular response and adaptation to environmental modifications. In this perspective, we compare and contrast the roles of SIRT1 and AMPK in several metabolic tissues and propose a working model of how the AMPK-SIRT1 axis may be regulated to control functions relevant to organismal physiology and pathophysiology.
Keywords: SIRT1, AMPK, Nampt, PGC1-α, Calorie Restriction, Starvation, Gluconeogenesis, Insulin
AMPK Regulation and Functions
AMPK is an evolutionary conserved energy sensor that regulates cellular metabolism.1 AMPK is a heterotrimeric protein kinase consisting of a catalytic subunit (α) and two regulatory subunits (β and γ).2 The γ subunit responds to an increased AMP to ATP ratio by inducing a conformational change that allows the phosphorylation of a threonine residue (Thr-172) within the activation domain of the α subunit, by the upstream kinases LKB13 and CaMKKβ (calmodulin –dependent protein kinase kinase β).4 Stimuli that activate AMPK are those that inhibit ATP production, including hypoxia, hyschemia, oxidative stresses, and glucose deprivation,5,2 or accelerate ATP consumption, such as rapid contraction of skeletal muscle.6 In addition, AMPK can be activated by the adipokines leptin and adiponectin, important regulators of whole–body energy metabolism.7 In general, activation of AMPK results in the repression of ATP-consuming anabolic processes (such as fatty acid, cholesterol, glycogen and protein synthesis, and gluconeogenesis) and activation of ATP-producing catabolic processes (such as fatty acid uptake and oxidation, and glucose uptake), to maintain cellular energy store.1, 8, 9 AMPK mediates these effects through the rapid phosphorylation of metabolic enzymes, such as acetyl CoA carboxylase (ACC)10 and hydroxymethylglutaryl-coA reductase (HMGR),11 two rate-limiting enzymes for fatty acid oxidation and cholesterol synthesis, respectively. If ATP stores remain depleted, AMPK can induce the phosphorylation of transcription factors and co-activators that regulate gene expression,12 including FoxO3,13 PGC1-α,14 p300,15 and HNF4.16 Interestingly, many of these transcription factors are also regulated by SIRT1.
SIRT1 Functions
SIRT1 is one of the seven mammalian orthologs (sirtuins SIRT1-7) of the yeast protein silent information regulator 2 (Sir2), a conserved NAD+-dependent protein deacetylase, that regulates life span extension and gene silencing in yeast.17, 18 SIRT1 catalyzes a reaction that couples lysine deacetylation to NAD+ hydrolysis19. During this reaction, NAD+ is hydrolyzed to nicotinamide (NAM) and O-acetyl-ADP ribose.20, 21 NAM is a strong inhibitor of SIRT1 deacetylase activity.22, 23 SIRT1 exerts its functions through deacetylation of target proteins, such as histones, transcription factors, and coregulators.24, 25 In lower organisms, such as yeast, worms and flies, Sir2 has been proposed to act as the molecular link between calorie restriction (CR) and longevity.26-31 However, results remain equivocal as to whether Sir2 is essential for the CR induced increase in life span.32, 33 Longo and colleagues have demonstrated that while Sir2 overexpression increases yeast replicative life span (defined as the number of daughter cells produced by a mother cell), it shortens the chronological life span (a measure of the length of time a yeast cell can survive in a non dividing status).34 In support of the hypothesis of Sir2 being an anti-aging protein, a number of studies has found that treatment with the natural polyphenolic compound resveratrol (RSV)-a SIRT1 activator35- significantly increased SIRT1 activity and, similarly to CR, extended life span of several species.35, 36 While compelling, these evidences should be interpreted with caution as RSV is not a specific activator of SIRT1 and has been found to act through the activation of several other pathways.37-39
CR is the only documented intervention that results in extension of life span in mammals, including human primates.40, 41 While the question remains as to whether this process is mediated via SIRT1,42 SIRT1 has been found to be activated by both CR and starvation in higher eukaryotes.43, 44 Indeed, SIRT1 is responsible for the increase in physical activity observed in mice undergoing CR45 and modulates gene expression in metabolically active tissues, such as skeletal muscle,46 liver,47, 48 and white adipose tissue (WAT)49 in response to lack of nutrients. Consistently, SIRT1 overexpressing transgenic mice share a number of characteristics with mice under CR: they are both leaner and metabolically more active, exhibit reduced blood cholesterol, insulin, and fasted glucose levels.50
In addition to the aforementioned metabolic functions, SIRT1 controls several other biological processes. SIRT1 regulates differentiation of cultured skeletal muscle cells,51and subsequent studies have implicated SIRT1 in numerous roles of mammalian cell physiology, from stress resistance,52 to cell differentiation of adypocites,49 endothelial cells,53neurons,54 and replicative senescence.55, 56 These effects of SIRT1 are believed to be mediated via deacetylation of transcription factors targets such as p53,55, 57 Forkhead box O (FoxO) proteins,52, 58, 59 p73, and E2F1.60 Interestingly, SIRT1-mediated deacetylation of these targets can either increase or decrease their transcriptional activity depending on the target gene. SIRT1 forms protein complexes with the acetyltransferases (HATs) PCAF, GCN5, and p300, and deacetylate them.51 As the autoacetylation of PCAF and p300 has regulatory functions,61, 62 the metabolic state of the cell may control the activity of the HATs via SIRT1. Indeed, deacetylation of the myogenic regulatory factor MyoD and its co-regulator PCAF have been found to inhibit muscle differentiation following SIRT1 over-expression (or activation) in muscle precursor cells51 (Figure 1), while SIRT1-mediated repression of the peroxisome proliferator-activated receptor γ PPARγ) and the nuclear receptor corepressor (NCoR) is associated with inhibition of adipogenesis.49
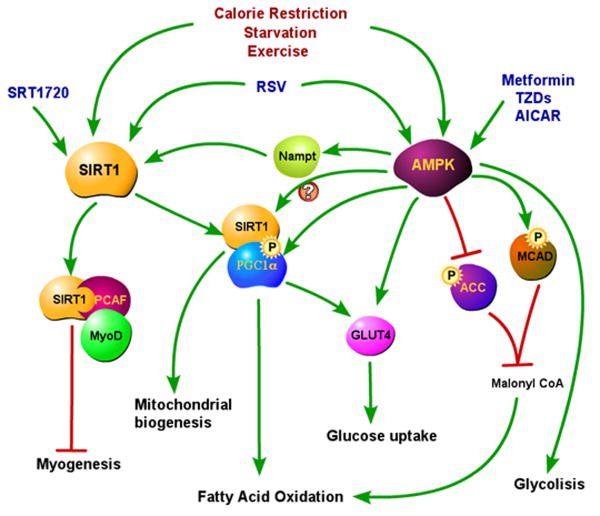
Figure 1. SIRT1 and AMPK in skeletal muscle.
Both SIRT1 and AMPK are activated by CR, starvation, exercise, and RSV. SRT1720 is a small molecule that specifically activates SIRT1, while AMPK is specifically activated by metformin and thiazolidinediones (TZDs). SIRT1 activation increases mitochondrial biogenesis and fatty acid oxidation through deacetylation and activation of PGC1-α. It is still not known yet whether PGC1-α induced expression of GLUT4 also requires SIRT1. Activated SIRT1 also blocks myogenesis, via MyoD and PCAF deacetylation.
AMPK activation increases fatty acid oxidation acutely by direct phosphorylation of the enzymes ACC and MCAD, and chronically by phosphorylation and activation of PGC1-α, that is also required for AMPK mediated increase in mitochondrial biogenesis. AMPK controls glucose uptake by increased translocation of GLUT4 to the membrane and by increasing GLUT4 expression through PGC1-α activation. AMPK may also activate PGC1-α indirectly through SIRT1 activation via increase of Nampt expression, as described in the pathway that blocks myogenesis. AMPK activation induces also glycolysis.
SIRT1 Regulation
The molecular mechanisms leading to SIRT1 activation have yet to be fully elucidated. Nonetheless, it is unlikely that a single, unifying rule underlies its activation or repression, as SIRT1 regulation seems to differ among the tissues and with the type of nutrient deprivation.63 Both chronic CR43 and acute fasting64 have been found to increase SIRT1 protein levels and activity. In addition, SIRT1 has been also found to be activated in response to oxidative stress44 and endurance exercise.65 Changes in SIRT1 protein levels have been shown to occur both at the transcriptional and post-transcriptional levels. For recent reviews of SIRT1 regulation, see refs 66, 67. The increase in the NAD+/NADH ratio induced by nutrient deprivation has been found to decrease the interaction of the redox sensor transcriptional corepressor CtBP (Carboxyl-terminal Binding Protein) with the transcriptional repressor, HIC1 (Hypermethylated in Cancer) which is subsequently released from the SIRT1 promoter,68 allowing SIRT1 transcription to proceed.69 In addition, the increase in SIRT1 transcription in response to acute nutrient withdrawal has been found to be regulated by FoxO3 and to require the presence of p53.64 Pyruvate, a metabolite increased in the liver of fasting animals, up-regulates hepatic SIRT1 protein levels without affecting its transcription,47 while glucose restriction in cultured myoblasts (undifferentiated muscle cells) increases SIRT1 activity without affecting its protein levels.70 Interestingly, when myotubes (differentiated muscle cells) are serum and glucose starved, both SIRT1 protein and mRNA levels are increased, a response similar to that observed in skeletal muscles of starved animals (our unpublished data).64 Therefore, it appears that the differentiation state of the cell may influence SIRT1 regulation in response to reduced nutrient availability. Whether this phenomenon is unique to muscle cells or applies also to other cell lineages remains to be investigated. To add further complexity to SIRT1 regulation, fasting and CR have been found to exert opposing effects on SIRT1 protein levels and activity in the liver. While fasting is associated with an increase,47 CR is associated with a decrease of SIRT1 protein in liver.63 These results are likely due to the changing redox state of the cells,69 given that fasting in the liver causes an increase in total NAD+,47 while CR decreases the NAD+/NADH ratio.63 Overall these findings indicate that the NAD+/NADH ratio is relevant to SIRT1 regulation. However, it remains unclear as to whether SIRT1 is regulated by the NAD+/NADH ratio,51 the absolute levels of NAD+,46, 47 NADH,71 NAM,72 or a thereof combination. Recent work has suggested a pivotal role for the NAD+ salvage pathway in regulating SIRT1 activity.73 The NAD+ salvage pathway regenerates NAD+ from its hydrolytic product NAM. While yeast, worms and flies require four steps to synthesize NAD+ from NAM, mammals require only two steps.74 In yeast, the rate-limiting enzyme of the NAD+ salvage pathway is the nicotinamidase PNC1, which deamidates NAM into nicotinic acid. PNC1 has been described to be activated by CR and stress, and has been suggested to increase replicative life span through activation of Sir2 75. Nicotinamide phosphoribosyltransferase (Nampt), also known as PBEF or visfatin, is the rate-limiting step of the mammalian NAD+ biosynthesis pathway,76 and catalyzes the conversion of NAM into nicotinamide mononucleotide (NMN). NMN is then processed by an adenylyltransferase to regenerate NAD+.77 Nampt has been proposed to be the functional homolog of the yeast PNC174 and, similarly to PNC1, is a stress and nutrient responsive gene,78 capable of modulating sirtuin activity.76 Nampt levels have been observed to increase in several cell lines in response to serum78 or glucose70 reduction, hypoxia, genotoxic stresses, as well as in the livers of starved rats78 and in the skeletal muscles of fasted mice.70 An extracellular form of Nampt (eNampt) has been recently documented and shown to work as a systemic NAD biosynthetic enzyme, able to convert circulating NAM into nicotinamide mononucleotide (NMN), the final product of Nampt reaction. The eNampt mediated systemic NAD biosynthesis has been shown so far to play a critical role for pancreatic β cell function,79 but it is tempting to speculate that it may critically affect other functions controlled by SIRT1.
Thus, signaling pathway/s influencing the levels of cellular, extracellular Nampt, or their enzymatic activity are likely to impact on SIRT1 activity.
Convergent Role of SIRT1 and AMPK in Aging
In addition to having common activatory events (glucose restriction, fasting, chronic calorie restriction, oxidative stress, and exercise), the AMPK and SIRT1 signaling pathways have similar effects on life span, aging and metabolism. Like SIRT1, AMPK has been proposed to be one of several molecules involved in regulating mammalian longevity.80 In support of this hypothesis, extra copies of the AMPK gene have been found to extend life span in C.elegans81 and mediate the effects of dietary restriction on longevity through the FoxO family of transcription factors.82 The aging process itself has been described to be associated with a decline in the activity of both SIRT1 and AMPK. SIRT1 protein (but not its mRNA) levels are diminished in mouse embryonic fibroblasts that exhibit premature senescence,83 while SIRT1 activity is reduced in cardiac (but not adipose) tissue of aged rates.84 Similarly to the effects of aging on SIRT1, AMPK phosphorylation decreases with aging, as indicated by findings demonstrating that AMPK activation by AICAR or exercise, is blunted in skeletal muscle of old rats.85 In the same study, old rats showed decreased mitochondrial biogenesis in response to chronic activation of AMPK with β-guanidinopropionic acid (β-GPA).
AMPK and SIRT1 in Skeletal Muscle Physiology
Skeletal muscle is the predominant site of insulin-induced glucose uptake and contributes to the maintenance of blood glucose levels in conditions of nutrient deprivation. In response to fasting, the liver increases glucose production (gluconeogenesis) while the muscle decreases glucose-consuming processes. In this process, muscle responds to a decrease of nutrient availability by switching from glucose to fatty acid utilization and increased mitochondrial biogenesis.
Fatty Acid Oxidation and Mitochondrial Biogenesis
Fatty acid oxidation in skeletal muscle involves a rate-limiting step regulated by carnitine palmitoyltransferase (CPT1). CPT1 allows long-chain acyl-CoA to move into the mitochondria, undergo β-oxidation, and enter the citric acid cycle to produce ATP. This process is allostericaly inhibited by malonyl-CoA,86 which is synthesized by acetyl-CoA carboxylase (ACC). AMPK has been found to increase fatty acid oxidation through inhibition of ACC, by direct phosphorylation.10As a consequence of ACC inhibition, malonyl-CoA concentrations are reduced and the inhibitory effect on CPT1 is relieved to promote long chain acyl-CoAs entry into the mitochondria.87 Malonyl-CoA levels are also negatively regulated by malonyl-CoA decarboxylase (MCD), the enzyme responsible for malonyl-CoA catabolism. Like ACC, MCD is also a direct AMPK substrate, and its phosphorylation results in activation and subsequent reduction in malonyl-CoA levels88 (Figure 1). In addition to these two regulatory pathways, AMPK appears also to positively control fatty oxidation through the transcriptional regulation of PPARα and PGC1-α.89 Following AMPK activation, increased expression of PGC1-α and PPARα promotes transcription of genes involved in mitochondrial fatty acid oxidation, including those for CPT1, fatty acid binding protein 3 (FABP3) and acyl-CoA oxidase (ACO).89
AMPK activation has also been found to increase mitochondrial biogenesis in skeletal muscle,90 and endurance capabilities in mice,91 in a process involving both PGC1-α90, 92 and the nuclear respiratory factor (NRF1).93 The AMPK agonist AICAR has been recently shown to induce metabolic genes and enhance running endurance, even in sedentary mice.91 While the mechanisms underlying these phenomena have yet to be fully elucidated, they may rely on the ability of nuclear AMPK complexes to directly phosphorylate transcriptional coregulators, such as PGC1-α (Figure 1). AMPK-induced PGC1-α phosporylation may initiate many of the gene regulatory functions of AMPK in skeletal muscle.14 Indeed, AMPK-dependent transcriptional regulation of glucose transporter 4 (Glut4), cytocrome C, and other mitochondrial genes is dependent on the presence of PGC1-α, both in cell culture and in vivo.14
PGC1-α-mediated transcription of genes involved in fatty acid oxidation and mitochondrial biogenesis requires SIRT1, at least in myotubes.46 SIRT1 has been shown to deacetylate and activate PGC1-α at the promoter regions of genes involved in fatty acid utilization (such as CPT1, MCAD, PGC1-α and PDK4) and mitochondrial respiration (such as cytocrome C and IDH3a).46 Thus, it appears conceivable that SIRT1 and AMPK converge on PGC1-α mediated signaling to regulate lipid oxidation and mitochondrial biogenesis.
In support of the proposed role of SIRT1 in regulating muscle metabolism in vivo, Lagouge and colleagues found that mice treated with RSV exhibited increased skeletal muscle mitochondrial function and were resistant to the increase in body mass and insulin resistance normally caused by a high fat diet (HFD).94 RSV counteracts these detrimental changes, at least in part, through the activation of SIRT1 and PGC1-α and downstream proteins including MCAD, cytocrome C, estrogen-related receptor alpha (ERR-α) and PGC1–α itself (Figure 1). Moreover, as observed in calorie restriction, animals treated with the SIRT1 agonist SRT1720 display increased skeletal muscle mitochondrial capacity.95 The development of muscle- specific SIRT1 transgenic animals will help in determining the role of this important protein in the metabolic response of muscle to low nutrients and high fat diet.
RSV-treated mice exhibited a reduced susceptibility to muscle fatigue that has been attributed to a switch in the muscle fiber phenotype, from fast (fatigue susceptible/glycolitic) type II fibers towards a slow (fatigue resistant/oxidative) type I phenotype.94 Similar changes in muscle fiber phenotype have been reported in skeletal muscle-tissue specific PGC1-α transgenic mice, which display a higher mitochondrial content and greater proportion of slow type I fibers.96 Interestingly, a shift towards a more oxidative phenotype in skeletal muscle in response to endurance exercise has been found to require AMPK97, 98 and to be promoted by administration of the AMPK activator AICAR.91 Given that both AMPK and PGC1-α activation can induce a shift from glycolytic to oxidative metabolism, it will be interesting to investigate whether a similar response is observed in muscle-specific SIRT1 transgenic mice.
Glucose Uptake
Glucose uptake into skeletal muscle occurs in an insulin-dependent and/or -independent manner.99 Exercise enhances muscle glucose uptake through an insulin-independent mechanism,100 a process that can be recapitulated via pharmacological activation of AMPK by AICAR.101-104 As skeletal muscle contraction leads to an increase in the creatine/phosphocreatine (an immediate energy pool) and AMP/ATP ratios,105 it has been hypothesized that AMPK activation may be responsible for the resultant increase in glucose transport. However, several recent experiments have indicated that AMPK activation may not be required for contraction-stimulated glucose transport.106-109 AMPK does appear to be required for AICAR-induced glucose transport.106 In addition, AMPK activation, either through exercise or AICAR administration, has been found to increase insulin sensitivity, resulting in a greater level of GLUT4 translocation and glucose transport following insulin release.110, 111
GLUT4 transcription appears to be regulated by PGC1-α in conjunction with the transcription factor MEF2C.112 In addition to its function in promoting GLUT4 membrane translocation, AMPK activation increases GLUT4 gene expression through PGC1-α phosphorylation14 and MEF2 nuclear translocation. 113
Whether SIRT1 is involved in skeletal muscle glucose uptake has yet to be examined. A recent study indicates that sirtuins may participate in this process, as RSV was able to increase glucose uptake, an effect independent of insulin and dependent on both AMPK and sirtuins.114 Interestingly, RSV did not affect GLUT4 membrane translocation, but rather increased the intrinsic activity of the glucose transporter.114 These results support the hypothesis that SIRT1 may play a role in controlling glucose uptake. However, further studies are required before a definitive role for SIRT1 in glucose uptake can be established
AMPK and SIRT1 in Liver Function
Gluconeogenesis
Under low nutrient conditions, glucose supply to metabolically active tissue, such as the brain and red blood cells, is maintained via hepatic glucose production, a process termed gluconeogenesis.
Gluconeogenesis in the liver is regulated through the function of numerous enzymes, including phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G-6-Pase). In response to fasting, glucagon is released by α-pancreatic cells and induces hepatic nuclear translocation of TORC2 (transducer of regulated CREB activity 2).115 Once in the nucleus, TORC2 binds to and activates the transcription factor CREB (CRE binding protein), which in turn stimulates gluconeogenesis and fatty acid oxidation through the induction of PGC1-α.116, 117 PGC1-α binds to FoxO1 and/or HNF4α and induces transcription of the gluoconeogenic genes such as PEPCK and G-6-Pase118, 119 (Figure 2). Under fed conditions, insulin is released to inhibit gluconeogenesis via the activation of Akt, which phosphorylates and inhibits several components of the gluconeogenic pathway including PGC1-α,120 FoxO1,121 and SIK2 (Salt Inducible Kinase2).122
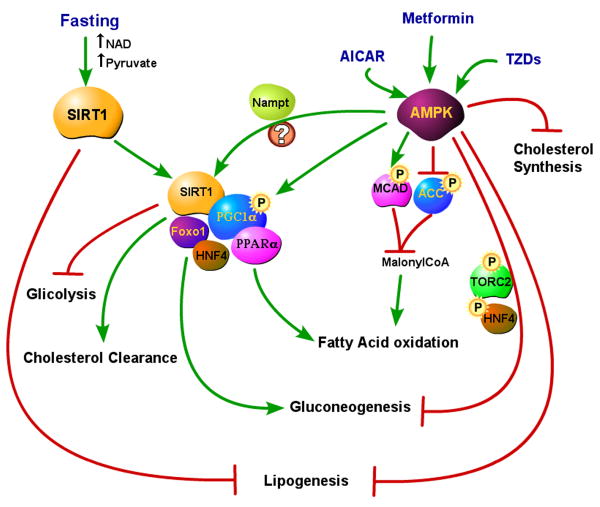
Figure 2. SIRT1 and AMPK in the fasting liver.
SIRT1 is activated in the fasting liver by the increases in NAD and pyruvate. In the fasted status, SIRT1 inhibits lipogenesis (likely through inhibition of SREBP-1c). In complex with PGC1–α, SIRT1 activates fatty acid oxidation (via PPARα) and gluconeogenesis (via Foxo1 and HNF4), and increases cholesterol clearance, while reducing glicolysis. AMPK activation by AICAR, metformin or TZDs blocks lipogenesis, cholesterol synthesis, and gluconeogenesis (the latter via TORC2 and HNF4 phosphorylation). In addition, AMPK activation induces fatty oxidation with the same mechanisms that operate in skeletal muscle. AMPK activation of PGC1-α (to increase the expression of genes involved in fatty acid oxidation) might also occur indirectly through SIRT1 activation via Nampt.
In parallel with hormonal regulation, PGC1-α activity in the liver is regulated by a direct nutrient signaling involving SIRT1.47 Increased NAD+ and pyruvate levels in the liver of fasted mice, resulted in an elevation of SIRT1 protein levels and deacetylase activity, subsequent upregulation of gluconeogenesis and downregulation of glycolytic pathways via PGC1-α deacetylation47 (Figure 2). Interestingly, the activation of SIRT1 signaling in the liver, in contrast to skeletal muscle, has not been found to increase mitochondrial biogenesis47. In a follow up study, this group also demonstrated that SIRT1 and PGC1-α induced gluconeogenesis in a co-dependent manner, such that PGC1-α was not able to stimulate glucose production in the absence of SIRT1 and vice versa48. Consistently, hepatic SIRT1 knockdown resulted in reduced response of gluconeogenic and fatty acid oxidation genes to fasting, and consequent mild hypoglycemia, decreased hepatic glucose output, increased glucose tolerance, and insulin sensitivity.48 Interestingly, SIRT1 liver-specific knockdown affected gluconeogenesis only in fasting conditions.48
Under extremely low intracellular ATP, or in response to stress signals, AMPK is activated to inhibit hepatic gluconeogenesis.2 AMPK inhibition of gluconeogenesis is believed to be predominantly mediated via the phosphorylation of TORC2, resulting in its cytoplasmic sequestration and subsequent inactivation of CREB.115 AMPK activation has also been proposed to inhibit gluconeogenesis via the phosphorylation of HNF4, another key regulator of gluconeogenesis16 (Figure 2). Importantly, AMPK inhibitory effect on gluconeogenesis can override the inductive signals initiated by glucagon and fasting. Thus, AMPK acts in a protective manner during low ATP conditions.115 Drugs that active AMPK, such as metformin,123 inhibit gluconeogenesis and lower glucose levels, and are employed for the treatment of type II diabetes.
The roles of AMPK and SIRT1 signaling in the regulation of gluconeogenesis during fasting conditions appear to be divergent with SIRT1 promoting and AMPK inhibiting glucose production (Figure 2). However, SIRT1 liver specific knock-out mice (LKO) have been found to be as capable as the control littermates, of up-regulating the gluconeogenic genes PEPCK, G6-Pase and PGC1-a, in response to CR, suggesting that SIRT1 does not control gluconeogenesis in a low calorie diet.63 In addition, Pfluger and colleagues124 have reported that mild whole-body overexpression of SIRT1 improves insulin sensitivity and glucose homeostasis of mice when fed a HFD, a response concluded to be mainly mediated via a reduction of glucose output from the liver. These results indicate that in high calorie conditions, SIRT1 may actually block hepatic gluconeogenesis. A decreased hepatic gluconeogenesis has been also shown to occur in Zucker fa/fa diabetic rats that had been treated with the specific SIRT1 activator SRT1720.95 A possible molecular mechanism of this anti-gluconeogenic effect of SIRT1 in no-starving conditions, could be via activation of LXR, as this nuclear receptor has been found to be able to inhibit gluconeogenesis125 and it is likely activated by SIRT1126 only in response to high calorie diet. Further experiments are required to clearly define the outcomes of SIRT1 activation on gluconeogenesis in several diet settings.
Cholesterol Metabolism and Lipogenesis
SIRT1 regulates cholesterol metabolism via deacetylation and subsequent activation of the nuclear receptor LXR and its targets ABCA1 (ATP-binding cassette transporter A1) and SREBP-1c126 (Figure 3). The nuclear receptor LXR enhances the reverse transport of cholesterol from peripheral tissues to the circulation by stimulating the expression of ABCA1, which directs cholesterol to apolipoprotein AI to form HDL (high-density lipoprotein).126 Consistently, whole-body and LKO SIRT1 mice exhibit reduced systemic HDL cholesterol levels in both the fed and fasted states.126 SIRT1 has been also found to favor hepatic cholesterol clearance through the activation of Cyp7A1, a rate limiting enzyme in bile acid synthesis.48
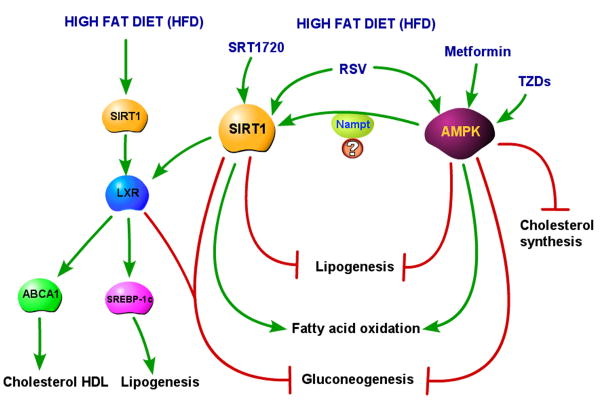
Figure 3. SIRT1 and AMPK in the liver during a high fat diet (HFD) regimen.
With a HFD, hepatic SIRT1 is required to activate LXR, which, by inducing SREBP-1c, increases lipogenesis. SIRT1-induced LXR activation promotes also the expression of LXR target ABCA1, which induces an increase in cholesterol HDL. When SIRT1 is activated by either RSV or SRT1720 in the animals fed a HFD, it blocks lipogenesis in the liver by reducing SREBP-1c and fatty acid synthase levels. In this condition, SIRT1 activation also reduces gluconeogenesis (likely through LXR activation) and increases fatty acid oxidation. The HFD setting does not change the outcomes of AMPK activation by metformin or TZDs. AMPK activation blocks lipogenesis, cholesterol synthesis and gluconeogenesis and increases fatty acid oxidation. The outcomes of SIRT1 and AMPK activation in the liver of HFD fed animals are coherent, and it is conceivable that some of the effects of AMPK might occur through SIRT1 activation via Nampt.
The repression of key lipogenic genes, such as FAS and SREBP-1c/ADD1, in response to fasting, has also been observed to be impaired in the liver of mice, whose SIRT1 protein was knocked down by infection with adenoviruses-expressing SIRT1 small hairpin RNA, suggesting that SIRT1 may contribute to the repression of lipogenesis in fasting conditions.48
In apparent contradiction is the report that hepatic SIRT1 seems necessary for the liver response to HFD.63 Chen and colleagues have found that weight gain and fat accumulation in WAT and liver induced by HFD are reduced in LKO SIRT1 mice. These mice are protected against the negative effects of HFD, exhibiting a higher glucose tolerance and lower blood glucose and insulin levels. The resistance of these mice to the detrimental effects of HFD has been proposed to be the result of lack of activation of the LXR target SREBP1c, and its target fatty acid synthase (FAS), which is the rate-limiting enzyme in fatty acid synthesis.63 These results indicate that SIRT1 is required to promote lipogenesis in the liver in response to HFD (Figure 3). However, mice on HFD and treated with either RSV or the SIRT1 agonist SRT1720, do not develop fatty liver, and even exhibit improved liver physiology and metabolic function95, 127 (Figure 3). Consistently, moderate overexpression of SIRT1 under the control of its native promoter, results in complete protection from hepatic steatosis due to a decreased lipid-induced inflammation and decreased lipogenesis (obtained via reduction of SREBP1c mRNA).124 AMPK has also been implicated in regulating hepatic lipogenesis, as well as lipid oxidation and cholesterol synthesis. As observed in skeletal muscle, AMPK activates hepatic fatty acid oxidation by direct phosphorylation and inhibition of ACC11 and activation of PGC1-α. AMPK also appears to decrease lipogenesis through downregulation of lipogenic genes, including SREBP-1, fatty acid synthase, and S14 (through inhibition of mRNA expression of SREBP-1)123, 128 and to inhibit cholesterol synthesis by phosphorylation and inactivation of hydroxymethylglutaryl-CoA reductase (HMGR), the rate limiting enzyme for cholesterol synthesis.129
Since both AMPK and SIRT1 have been found to repress lipogenesis by reducing SREBP-1 levels123, 128 (Figure 3), they may be components of the same pathway. AMPK and SIRT1 may be part of an autoregulatory loop where AMPK activates SIRT170 and SIRT1 feeds-back to activate AMPK,130 likely via LKB1 deacetylation and activation.131 Further experiments are required to clarify these emerging complex interactions between the AMPK and SIRT1 pathways.
AMPK and SIRT1 in Adipose Tissues and Pancreatic β Cells
Adipose tissue
Adipose tissue is a major site of triglyceride (TG) storage and therefore becomes an important source of energy when glucose availability is limited during fasting and starvation. Under these conditions, adipose TG stores are mobilized to give rise to free fatty acids (FFA), which can then be utilized by other tissues for energy production. SIRT1 has been found to suppress adipocyte differentiation and to prevent TG accumulation in white adipose tissue through repression of PPARγ.49 Similarly, AMPK activation inhibits adipocyte differentiation132 and lipogenesis via increased ACC phosphorylation.133 SIRT1 has also been proposed to increase lipolysis and TG mobilization in response to fasting,49 however the mechanisms underlying this effect have to be fully elucidated. Whether AMPK activates or inhibits lipolysis remains controversial.134
Pancreatic β Cells
Pancreatic β cells play a central role in maintaining glucose homeostasis by secreting insulin in response to elevated blood glucose levels. Following a meal glucose enters the pancreatic β cells via the GLUT2 transporter and is then metabolized during glycolysis, with a subsequent increase in intracellular ATP/ADP ratio. The increase in ATP results in the closure of ATP-sensitive potassium channels, causing membrane depolarization and opening of the voltage-gated Ca2+ channels. The influx of Ca2+ ions is a critical step for the release of insulin from the pancreatic β cells.135, 136
A physiological role of SIRT1 in insulin secretion has been demonstrated in β-cell-specific-SIRT1-overexpressing (BESTO) mice and in SIRT1 knock-out animals. BESTO mice exhibit improved glucose tolerance and enhanced insulin secretion in response to glucose administration.137 In contrast, SIRT1 knock out animals have lower levels of circulating insulin.138 Similarly, the ability to release insulin is blunted in pancreatic β cells whose SIRT1 levels have been reduced by siRNA.138 SIRT1 has been proposed to enhance insulin secretion through the suppression of uncoupling protein 2 (UCP2) expression,138, 137 UCP2 levels are increased upon food deprivation to prevent the release of insulin. As SIRT1 favors insulin secretion through UCP2 repression,137 it has been postulated that SIRT1 expression in pancreatic β cells must be activated in response to a high glucose meal and inhibited by starvation, a response in direct opposition to that observed in other metabolic tissues, such as skeletal muscle and WAT. In support of this hypothesis, Guarente and colleagues138 have demonstrated that in response to overnight fasting, total NAD+ levels in the pancreas are reduced, suggesting that SIRT1 activity is likely reduced.
eNampt and Insulin Release
In addition to SIRT1, insulin release has also been found to be positively regulated by the extracellular form of Nampt (eNampt).79 Haplodeficiency of Nampt causes defects in NAD+ biosynthesis and is associated with impaired glucose stimulated insulin secretion in pancreatic islets. Interestingly, these defects can be corrected by administration of nicotinamide mononucleotide (NMN), the final product of the Nampt reaction in the NAD+ salvage pathway.79 Extracellular Nampt therefore appears to act as a systemic NAD+ biosynthetic enzyme that converts circulating levels of NAM into NMN, which can then be taken up into peripheral tissues and converted back into NAD+. This pathway is likely to be extremely important in tissues such as pancreatic β cells, where the levels of endogenous Nampt are very low.79 While intracellular Nampt has been described to increase in vivo in response to starvation in several tissues,70, 78 very little is known about the regulation of eNampt. Increased circulating levels of visfatin (eNampt) have been correlated to several pathological conditions in humans, such as adiposity,139 diabetes,140, 141 metabolic syndrome,142 and inflammatory diseases,143 but results from these studies have not confirmed whether the increase in visfatin levels is a protective compensatory mechanism, or a pathogenic cause in the development of such diseases.144, 145 It will therefore be important for future studies to determine the physiological regulation of eNampt and NMN levels under normal and pathological conditions. As eNampt/visfatin levels have been found to increase after i.v. infusion of glucose in healthy individuals,146 it is tempting to speculate that eNampt and subsequently NMN levels may be regulated by the feeding status, such as upregulation in response to feeding and downregulation upon fasting/starvation. This response may be relevant to regulate SIRT1 activity in tissues, such as the pancreatic β-cells, that contain undetectable levels of the intracellular Nampt (Figure 4). Deregulation of the eNampt secretory pathway may also provide an explanation for the hyperinsulinemia observed in the metabolic syndrome or in the initial phases of diabetes.
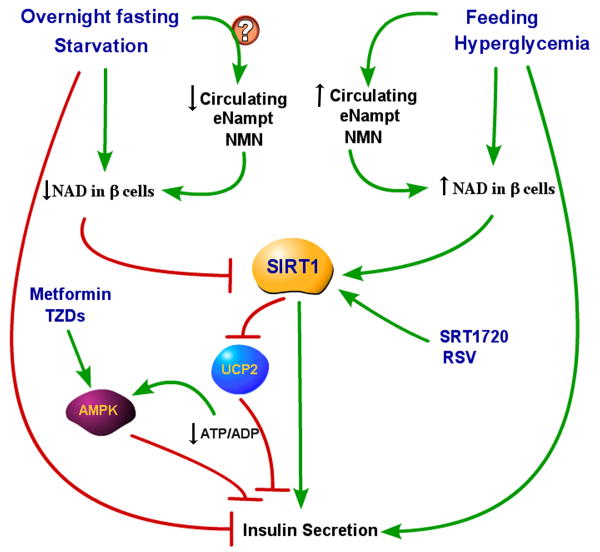
Figure 4. eNampt, SIRT1 and AMPK in the control of insulin secretion.
Insulin secretion by β pancreatic cells is controlled by the nutritional status and subsequent glucose circulating levels, with fasting/starvation inhibiting insulin secretion and feeding increasing it. While glucose itself directly controls the release of insulin from pancreatic cells, insulin release has been also found to be partly controlled by circulating eNampt and NMN levels and by SIRT1 activity in pancreatic β cells. We propose a unifying model where SIRT1 activity in β cells may be controlled by the circulating eNampt and NMN levels through their influence on intracellular NAD in β cells. In response to starvation or overnight fasting, circulating levels of eNampt and NMN are expected to be decreased, with subsequent reduction of NAD levels in β cells and inhibition of SIRT1 activity. UCP2 levels are then allowed to increase, causing a reduction of ATP/ADP ratio and inhibition of insulin release. The reduction of ATP/ADP may also activate AMPK, that in turn, contributes to the insulin release block. In a high glucose levels conditions, such as after a meal or glucose infusion, circulating levels of eNampt (and likely NMN) are increased. The supposedly increased NMN influx into pancreatic β cells is expected to increase NAD levels and consequently SIRT1 activity, therefore favoring insulin release, via SIRT1 mediated repression of UCP2.
AMPK and Insulin Release
In the pancreas, AMPK and SIRT1 signal in a divergent manner, such that SIRT1 favors insulin release while AMPK inhibits it.147-149 The increase in ATP levels in pancreatic β cells as a consequence of high glucose levels has been found to repress AMPK activity, while under low glucose conditions, the decrease in ATP levels has been demonstrated to activate AMPK, which then inhibits glucose stimulated insulin release.147-149 This inhibition of insulin release may appear as a detrimental response, particularly in light of the use of pharmacological AMPK agonists for the treatment of diabetes. However, the AMPK-induced inhibition of insulin hypersecretion may be teleologically interpreted as an attempt to prevent pancreatic exhaustion and protect from lipotoxicity, through increased fatty acid oxidation in pancreatic β cells.
AMPK and SIRT1 as Molecular Targets of Aging-Related Diseases
SIRT1 and Insulin Resistance
SIRT1 has recently been identified as having a protective role in skeletal muscle by preventing insulin resistance in this tissue. SIRT1 expression or its activation by RSV has been found to improve insulin sensitivity of insulin resistant muscle cells, via repression of the protein tyrosine phosphatase PTP1B.150 PTP1B negatively regulates the insulin signal transduction cascade, through direct dephosphorylation of the insulin receptor, thus SIRT1 would be expected to increase the downstream signaling response to insulin receptor binding. It appears likely that PTP1B repression is not the sole mechanism by which SIRT1 increases insulin sensitivity, as SIRT1 is also associated with increased fatty acid oxidation and mitochondrial biogenesis. Li and colleagues have found that SIRT1 deacetylates the insulin receptor substrate 2 (IRS2) favoring IGF1 signaling in neuronal cells.151 Future studies are required to determine whether a similar response also occurs in skeletal muscle.
Insulin resistant tissues and insulin resistant cells show decreased levels of SIRT1.150 It will be of interest to evaluate whether SIRT1 down-regulation in insulin resistant tissues occurs at the transcriptional or post-translational levels and whether it represents a consequence or it plays a causative role in the induction of insulin resistance.
Small Molecules that Activate SIRT1 and AMPK for Treatment of Metabolic Disorders
Compounds that activate SIRT1 have been proposed as useful pharmacological agents to treat diabetes.25 Promising results have been observed with new small molecules activators of SIRT1, such as SRT1720, which have been found to be effective for treating diabetes and/or insulin resistance in several animal models.95
While there had been initial concerns regarding the potential of SIRT1 activation to induce gluconeogenesis in the liver of diabetic patients, studies using both RSV and SRT1720 have indicated that this is not the case under normal feeding conditions.94, 95 AMPK is a target of commonly used anti-diabetic drugs, such as the biguanide metformin152 or the thiazolidinedione family (TZDs).153 The mechanism of action of metformin has been well described and involves decreasing blood glucose levels predominantly via the suppression of hepatic glucose production, and to a lesser extent, via increasing skeletal muscle glucose uptake.154 The effects exerted by AMPK and SIRT1 on metabolism raises the question of whether and in which pathological conditions it may be beneficial to combine SIRT1 and AMPK activators to lower blood glucose levels, increase insulin sensitivity and improve the lipid profile of diabetic patients.
A Molecular Model for an Activated AMPK-SIRT1 Axis
It is by now clear that AMPK, SIRT1, and Nampt are intertwined in regulating different metabolic processes. The existence of a mechanistic relationship between these three proteins has emerged during an investigation of the effects of glucose restriction (GR) on the differentiation of cultured skeletal muscle cells. Skeletal myoblasts exposed to low glucose exhibit impaired differentiation through activation of a pathway that targets the enzymatic activity of SIRT1.70 AMPK was shown to act upstream of SIRT1 and to mediates its activation via increased transcription of the NAD+ salvage enzyme Nampt. The effects exerted by AMPK activation on cell differentiation were dependent on both SIRT1 and Nampt. The AMPK induced expression of Nampt resulted in an increase in the intracellular NAD+/NADH ratio and a decrease of NAM levels. These changes in the redox state and in the intracellular NAM levels have been shown to lead to the activation of SIRT1 and the subsequent inhibition of muscle gene expression via MyoD, PCAF and histone H4 deacetylation.51 Whether the increase in the NAD+/NADH ratio or the decrease of NAM is equally important in the regulation of SIRT1 activity, remains inconclusive. However, the reduction of NAM levels without modifying the NAD+/NADH ratio, via the expression of the enzyme nicotinamide methyltransferase (NNMT), has been found to be sufficient to recapitulate the effects obtained by SIRT1,75 suggesting that NAM may be the predominant mediator of SIRT1 activity. Skeletal muscle expression of a number of AMPK targets (UCP2, UCP3, and PDK4) is increased during fasting in adult mice and such an increase was reduced in SIRT1 knock-out mice,70 suggesting that AMPK and SIRT1 may coherently regulate a specific metabolic gene expression program in the skeletal muscle of adult mice. However, while fasting-induced activation of AMPK targets requires the presence of SIRT1, under normocaloric conditions the same AMPK targets appeared to be negatively regulated by SIRT1.70 Several mechanisms-including modulation of SIRT1 activity or SIRT1-associated factors- may account for functionally switching SIRT1 from a repressor to an activator upon AMPK activation. It is evident that if this model (AMPK>Nampt>SIRT1) satisfactorily explains cellular processes where the individual protein components function coherently, its fails when AMPK and SIRT1 have opposite outcomes. In these situations, AMPK and SIRT1 modifiers are likely involved in modulating their activities.
Further studies will be required to define pathways in which AMPK and SIRT1 function coherently. The treatment of various tissue- specific knock out of SIRT1 with AMPK activators would be of great help in discriminating which of the multiple AMPK functions require SIRT1 and vice versa.
Muscle Regeneration: a Role for AMPK and SIRT1?
The AMPK-SIRT1 pathway may be physiologically relevant in vivo for the activation, proliferation and differentiation of satellite cells. Satellite cells are responsible for postnatal muscle growth and for muscle regeneration in response to muscle injury and/or exercise.155, 156 While an AMPK function in these cells has not been addressed yet, SIRT1 has been shown to regulate myogenesis in culture. By opposing muscle cell differentiation,51 SIRT1 may, by default, favor satellite cell proliferation. Impaired or reduced satellite cell proliferation in the absence of SIRT1 could provide an explanation for the smaller muscles observed in the SIRT1 knock-out mice.157 Given that both SIRT1 and AMPK activities decrease during normal aging, it is also tempting to speculate that the decreased SIRT1 and/or AMPK activities in satellite cells might be partially responsible for their decrease in number and function during aging,158, 159 perhaps via synergistic interaction with the Notch signaling pathway.160 Should a role for SIRT1 be confirmed in vivo, SIRT1 agonists and antagonists may found an application in the treatment of muscle damage and sarcopenia. SIRT1 agonists may be employed in the initial phases of muscle injury to amplify expansion of the satellite cell reservoir followed by administration of SIRT1 inhibitors to favor differentiation of the expanded cell population.
Conclusions
Depending on the initial stimulus and the target tissue, AMPK and SIRT1 display concordant or divergent regulatory functions. While in skeletal muscle they seem to coherently regulate metabolic processes (fatty acid oxidation, mitochondrial biogenesis, glucose uptake), their roles diverge when considering hepatic glucose production in response to energy deprivation and in the insulin release from pancreatic β cells. Therefore, the potential benefits of a co-administration of AMPK and SIRT1 activators for the therapy of type II diabetes or other metabolic disorders need to be evaluated with these considerations in mind. Moreover, further studies are needed to examine the negative effects of chronic SIRT1 activation, as recent reports have unveiled a pro-aging role of SIRT1 in the brain.151 Considerable research efforts are needed to better understand the multiple and intercrossing roles of AMPK and SIRT1 pathways in regulating metabolic processes in each tissue in response to different stimuli.
Acknowledgments
We would like to thank James Ryall (NIAMS, NIH) for discussion and critical reading of the manuscript. This work was supported by the Intramural Research Program of the National Institute of Arthritis, Musculoskeletal, and Skin Diseases of the National Institutes of Health.
References
- 1.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–85. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 2.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–83. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–35. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Winder WW, Thomson DM. Cellular energy sensing and signaling by AMP-activated protein kinase. Cell Biochem Biophys. 2007;47:332–47. doi: 10.1007/s12013-007-0008-7. [DOI] [PubMed] [Google Scholar]
- 6.Jorgensen SB, Rose AJ. How is AMPK activity regulated in skeletal muscles during exercise? Front Biosci. 2008;13:5589–604. doi: 10.2741/3102. [DOI] [PubMed] [Google Scholar]
- 7.Hardie DG. The AMP-activated protein kinase pathway--new players upstream and downstream. J Cell Sci. 2004;117:5479–87. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 8.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–41. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg GR, Macaulay SL, Febbraio MA, Kemp BE. AMP-activated protein kinase--the fat controller of the energy railroad. Can J Physiol Pharmacol. 2006;84:655–65. doi: 10.1139/y06-005. [DOI] [PubMed] [Google Scholar]
- 10.Winder WW, Wilson HA, Hardie DG, Rasmussen BB, Hutber CA, Call GB, et al. Phosphorylation of rat muscle acetyl-CoA carboxylase by AMP-activated protein kinase and protein kinase A. J Appl Physiol. 1997;82:219–25. doi: 10.1152/jappl.1997.82.1.219. [DOI] [PubMed] [Google Scholar]
- 11.Schimmack G, Defronzo RA, Musi N. AMP-activated protein kinase: Role in metabolism and therapeutic implications. Diabetes Obes Metab. 2006;8:591–602. doi: 10.1111/j.1463-1326.2005.00561.x. [DOI] [PubMed] [Google Scholar]
- 12.McGee SL, Hargreaves M. AMPK and transcriptional regulation. Front Biosci. 2008;13:3022–33. doi: 10.2741/2907. [DOI] [PubMed] [Google Scholar]
- 13.Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282:30107–19. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 14.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC1-alpha. Proc Natl Acad Sci U S A. 2007;104:12017–22. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leff T. AMP-activated protein kinase regulates gene expression by direct phosphorylation of nuclear proteins. Biochem Soc Trans. 2003;31:224–7. doi: 10.1042/bst0310224. [DOI] [PubMed] [Google Scholar]
- 16.Hong YH, Varanasi US, Yang W, Leff T. AMP-activated protein kinase regulates HNF4alpha transcriptional activity by inhibiting dimer formation and decreasing protein stability. J Biol Chem. 2003;278:27495–501. doi: 10.1074/jbc.M304112200. [DOI] [PubMed] [Google Scholar]
- 17.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 18.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–35. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 19.Tanny JC, Moazed D. Coupling of histone deacetylation to NAD breakdown by the yeast silencing protein Sir2: Evidence for acetyl transfer from substrate to an NAD breakdown product. Proc Natl Acad Sci U S A. 2001;98:415–20. doi: 10.1073/pnas.031563798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borra MT, O'Neill FJ, Jackson MD, Marshall B, Verdin E, Foltz KR, et al. Conserved enzymatic production and biological effect of O-acetyl-ADP-ribose by silent information regulator 2-like NAD+-dependent deacetylases. J Biol Chem. 2002;277:12632–41. doi: 10.1074/jbc.M111830200. [DOI] [PubMed] [Google Scholar]
- 21.Tanner KG, Landry J, Sternglanz R, Denu JM. Silent information regulator 2 family of NAD- dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose. Proc Natl Acad Sci U S A. 2000;97:14178–82. doi: 10.1073/pnas.250422697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277:45099–107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- 23.Sauve AA, Schramm VL. Sir2 regulation by nicotinamide results from switching between base exchange and deacetylation chemistry. Biochemistry. 2003;42:9249–56. doi: 10.1021/bi034959l. [DOI] [PubMed] [Google Scholar]
- 24.Feige JN, Johan A. Transcriptional targets of sirtuins in the coordination of mammalian physiology. Curr Opin Cell Biol. 2008;20:303–9. doi: 10.1016/j.ceb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang WJ. Sirtuins: Novel targets for metabolic disease in drug development. Biochem Biophys Res Commun. 2008 doi: 10.1016/j.bbrc.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 26.Guarente L. Sir2 links chromatin silencing, metabolism, and aging. Genes Dev. 2000;14:1021–6. [PubMed] [Google Scholar]
- 27.Guarente L. Sirtuins in aging and disease. Cold Spring Harb Symp Quant Biol. 2007;72:483–8. doi: 10.1101/sqb.2007.72.024. [DOI] [PubMed] [Google Scholar]
- 28.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–8. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 29.Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–8. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 30.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–30. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 31.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–6003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaeberlein M, Powers RW., 3rd Sir2 and calorie restriction in yeast: a skeptical perspective. Ageing Res Rev. 2007;6:128–40. doi: 10.1016/j.arr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Smith DL, Jr, McClure JM, Matecic M, Smith JS. Calorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently of the Sirtuins. Aging Cell. 2007;6:649–62. doi: 10.1111/j.1474-9726.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- 34.Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, McGrew K, et al. Sir2 blocks extreme life-span extension. Cell. 2005;123:655–67. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 35.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–6. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 36.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–9. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 37.Saiko P, Szakmary A, Jaeger W, Szekeres T. Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat Res. 2008;658:68–94. doi: 10.1016/j.mrrev.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Orallo F. Trans-resveratrol: a magical elixir of eternal youth? Curr Med Chem. 2008;15:1887–98. doi: 10.2174/092986708785132951. [DOI] [PubMed] [Google Scholar]
- 39.Pirola L, Frojdo S. Resveratrol: one molecule, many targets. IUBMB Life. 2008;60:323–32. doi: 10.1002/iub.47. [DOI] [PubMed] [Google Scholar]
- 40.Roth GS, Ingram DK, Lane MA. Caloric restriction in primates and relevance to humans. Ann N Y Acad Sci. 2001;928:305–15. doi: 10.1111/j.1749-6632.2001.tb05660.x. [DOI] [PubMed] [Google Scholar]
- 41.Lane MA, Black A, Handy A, Tilmont EM, Ingram DK, Roth GS. Caloric restriction in primates. Ann N Y Acad Sci. 2001;928:287–95. doi: 10.1111/j.1749-6632.2001.tb05658.x. [DOI] [PubMed] [Google Scholar]
- 42.Guarente L. Calorie restriction and SIR2 genes--towards a mechanism. Mech Ageing Dev. 2005;126:923–8. doi: 10.1016/j.mad.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–2. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 44.Wang F, Nguyen M, Qin FX, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007;6:505–14. doi: 10.1111/j.1474-9726.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 45.Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- 46.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC1-alpha. Embo J. 2007;26:1913–23. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC1-alpha and SIRT1. Nature. 2005;434:113–8. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 48.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A. 2007;104:12861–6. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–6. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007 doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 51.Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, et al. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- 52.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 53.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–58. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schroter F, Ninnemann O, et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10:385–94. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- 55.Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, Minucci S, et al. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. Embo J. 2002;21:2383–96. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ota H, Akishita M, Eto M, Iijima K, Kaneki M, Ouchi Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol. 2007;43:571–9. doi: 10.1016/j.yjmcc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 57.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–48. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 58.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, et al. Mammalian SIRT1 Represses Forkhead Transcription Factors. Cell. 2004;116:551–63. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 59.Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K, et al. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med. 2005;16:237–43. [PubMed] [Google Scholar]
- 60.Wang C, Chen L, Hou X, Li Z, Kabra N, Ma Y, et al. Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat Cell Biol. 2006;8:1025–31. doi: 10.1038/ncb1468. [DOI] [PubMed] [Google Scholar]
- 61.Santos-Rosa H, Valls E, Kouzarides T, Martinez-Balbas M. Mechanisms of P/CAF auto-acetylation. Nucleic Acids Res. 2003;31:4285–92. doi: 10.1093/nar/gkg655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson PR, Wang D, Wang L, Fulco M, Pediconi N, Zhang D, et al. Regulation of the p300 HAT domain via a novel activation loop. Nat Struct Mol Biol. 2004;11:308–15. doi: 10.1038/nsmb740. [DOI] [PubMed] [Google Scholar]
- 63.Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, et al. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;22:1753–7. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–8. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 65.Suwa M, Nakano H, Radak Z, Kumagai S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor gamma coactivator-1alpha protein expressions in rat skeletal muscle. Metabolism. 2008;57:986–98. doi: 10.1016/j.metabol.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 66.Kwon HS, Ott M. The ups and downs of SIRT1. Trends Biochem Sci. 2008 doi: 10.1016/j.tibs.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 67.Zschoernig B, Mahlknecht U. SIRTUIN 1: Regulating the regulator. Biochem Biophys Res Commun. 2008 doi: 10.1016/j.bbrc.2008.08.137. [DOI] [PubMed] [Google Scholar]
- 68.Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437–48. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Q, Wang SY, Fleuriel C, Leprince D, Rocheleau JV, Piston DW, et al. Metabolic regulation of SIRT1 transcription via a HIC1:CtBP corepressor complex. Proc Natl Acad Sci U S A. 2007;104:829–33. doi: 10.1073/pnas.0610590104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–73. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–6. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sauve AA, Moir RD, Schramm VL, Willis IM. Chemical activation of Sir2-dependent silencing by relief of nicotinamide inhibition. Mol Cell. 2005;17:595–601. doi: 10.1016/j.molcel.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 73.Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Cohen H, Lin SS, et al. Manipulation of a nuclear NAD+ salvage pathway delays aging without altering steady-state NAD+ levels. J Biol Chem. 2002;277:18881–90. doi: 10.1074/jbc.M111773200. [DOI] [PubMed] [Google Scholar]
- 74.Yang H, Lavu S, Sinclair DA. Nampt/PBEF/Visfatin: a regulator of mammalian health and longevity? Exp Gerontol. 2006;41:718–26. doi: 10.1016/j.exger.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–5. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–63. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 77.Revollo JR, Grimm AA, Imai S. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Curr Opin Gastroenterol. 2007;23:164–70. doi: 10.1097/MOG.0b013e32801b3c8f. [DOI] [PubMed] [Google Scholar]
- 78.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, et al. Nutrient-Sensitive Mitochondrial NAD(+) Levels Dictate Cell Survival. Cell. 2007;130:1095–107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, et al. Nampt/PBEF/Visfatin Regulates Insulin Secretion in beta Cells as a Systemic NAD Biosynthetic Enzyme. Cell Metab. 2007;6:363–75. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCarty MF. Chronic activation of AMP-activated kinase as a strategy for slowing aging. Med Hypotheses. 2004;63:334–9. doi: 10.1016/j.mehy.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 81.Apfeld J, O'Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18:3004–9. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, et al. An AMPK-FOXO Pathway Mediates Longevity Induced by a Novel Method of Dietary Restriction in C. elegans. Curr Biol. 2007 doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sasaki T, Maier B, Bartke A, Scrable H. Progressive loss of SIRT1 with cell cycle withdrawal. Aging Cell. 2006;5:413–22. doi: 10.1111/j.1474-9726.2006.00235.x. [DOI] [PubMed] [Google Scholar]
- 84.Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, et al. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11:139–50. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- 85.Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, et al. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5:151–6. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruderman NB, Dean D. Malonyl CoA, long chain fatty acyl CoA and insulin resistance in skeletal muscle. J Basic Clin Physiol Pharmacol. 1998;9:295–308. doi: 10.1515/jbcpp.1998.9.2-4.295. [DOI] [PubMed] [Google Scholar]
- 87.Roepstorff C, Halberg N, Hillig T, Saha AK, Ruderman NB, Wojtaszewski JF, et al. Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am J Physiol Endocrinol Metab. 2005;288:E133–42. doi: 10.1152/ajpendo.00379.2004. [DOI] [PubMed] [Google Scholar]
- 88.Saha AK, Schwarsin AJ, Roduit R, Masse F, Kaushik V, Tornheim K, et al. Activation of malonyl-CoA decarboxylase in rat skeletal muscle by contraction and the AMP-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-beta -D-ribofuranoside. J Biol Chem. 2000;275:24279–83. doi: 10.1074/jbc.C000291200. [DOI] [PubMed] [Google Scholar]
- 89.Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ, Oh KS, et al. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC1- Biochem Biophys Res Commun. 2006;340:291–5. doi: 10.1016/j.bbrc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 90.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–7. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–15. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol. 2006;574:33–9. doi: 10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, et al. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281:E1340–6. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- 94.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC1-alpha. Cell. 2006;127:1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 95.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–6. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, et al. Transcriptional co-activator PGC1- alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 97.Suwa M, Nakano H, Kumagai S. Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC1- in rat muscles. J Appl Physiol. 2003;95:960–8. doi: 10.1152/japplphysiol.00349.2003. [DOI] [PubMed] [Google Scholar]
- 98.Rockl KS, Hirshman MF, Brandauer J, Fujii N, Witters LA, Goodyear LJ. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes. 2007;56:2062–9. doi: 10.2337/db07-0255. [DOI] [PubMed] [Google Scholar]
- 99.Krook A, Wallberg-Henriksson H, Zierath JR. Sending the signal: molecular mechanisms regulating glucose uptake. Med Sci Sports Exerc. 2004;36:1212–7. doi: 10.1249/01.mss.0000132387.25853.3b. [DOI] [PubMed] [Google Scholar]
- 100.Hayashi T, Wojtaszewski JF, Goodyear LJ. Exercise regulation of glucose transport in skeletal muscle. Am J Physiol. 1997;273:E1039–51. doi: 10.1152/ajpendo.1997.273.6.E1039. [DOI] [PubMed] [Google Scholar]
- 101.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:E1107–12. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 102.Bergeron R, Russell RR, 3rd, Young LH, Ren JM, Marcucci M, Lee A, et al. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am J Physiol. 1999;276:E938–44. doi: 10.1152/ajpendo.1999.276.5.E938. [DOI] [PubMed] [Google Scholar]
- 103.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–73. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- 104.Musi N, Hayashi T, Fujii N, Hirshman MF, Witters LA, Goodyear LJ. AMP-activated protein kinase activity and glucose uptake in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2001;280:E677–84. doi: 10.1152/ajpendo.2001.280.5.E677. [DOI] [PubMed] [Google Scholar]
- 105.Winder WW. Energy-sensing and signaling by AMP-activated protein kinase in skeletal muscle. J Appl Physiol. 2001;91:1017–28. doi: 10.1152/jappl.2001.91.3.1017. [DOI] [PubMed] [Google Scholar]
- 106.Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, et al. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside but not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–9. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- 107.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–94. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 108.Fujii N, Hirshman MF, Kane EM, Ho RC, Peter LE, Seifert MM, et al. AMP-activated protein kinase alpha2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem. 2005;280:39033–41. doi: 10.1074/jbc.M504208200. [DOI] [PubMed] [Google Scholar]
- 109.Koh HJ, Brandauer J, Goodyear LJ. LKB1 and AMPK and the regulation of skeletal muscle metabolism. Curr Opin Clin Nutr Metab Care. 2008;11:227–32. doi: 10.1097/MCO.0b013e3282fb7b76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jessen N, Pold R, Buhl ES, Jensen LS, Schmitz O, Lund S. Effects of AICAR and exercise on insulin-stimulated glucose uptake, signaling, and GLUT-4 content in rat muscles. J Appl Physiol. 2003;94:1373–9. doi: 10.1152/japplphysiol.00250.2002. [DOI] [PubMed] [Google Scholar]
- 111.Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab. 2002;282:E18–23. doi: 10.1152/ajpendo.2002.282.1.E18. [DOI] [PubMed] [Google Scholar]
- 112.Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, et al. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC1- Proc Natl Acad Sci U S A. 2001;98:3820–5. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holmes BF, Sparling DP, Olson AL, Winder WW, Dohm GL. Regulation of muscle GLUT4 enhancer factor and myocyte enhancer factor 2 by AMP-activated protein kinase. Am J Physiol Endocrinol Metab. 2005;289:E1071–6. doi: 10.1152/ajpendo.00606.2004. [DOI] [PubMed] [Google Scholar]
- 114.Breen DM, Sanli T, Giacca A, Tsiani E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem Biophys Res Commun. 2008 doi: 10.1016/j.bbrc.2008.06.104. [DOI] [PubMed] [Google Scholar]
- 115.Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–11. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 116.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC1- Nature. 2001;413:179–83. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 117.Louet JF, Hayhurst G, Gonzalez FJ, Girard J, Decaux JF. The coactivator PGC1- is involved in the regulation of the liver carnitine palmitoyltransferase I gene expression by cAMP in combination with HNF4 alpha and cAMP-response element-binding protein (CREB) J Biol Chem. 2002;277:37991–8000. doi: 10.1074/jbc.M205087200. [DOI] [PubMed] [Google Scholar]
- 118.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6:208–16. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 119.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC1- Nature. 2001;413:131–8. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 120.Li X, Monks B, Ge Q, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC1-alpha transcription coactivator. Nature. 2007;447:1012–6. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- 121.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 122.Dentin R, Liu Y, Koo SH, Hedrick S, Vargas T, Heredia J, et al. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449:366–9. doi: 10.1038/nature06128. [DOI] [PubMed] [Google Scholar]
- 123.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Laffitte BA, Chao LC, Li J, Walczak R, Hummasti S, Joseph SB, et al. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc Natl Acad Sci U S A. 2003;100:5419–24. doi: 10.1073/pnas.0830671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 Deacetylates and Positively Regulates the Nuclear Receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 127.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kohjima M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, et al. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int J Mol Med. 2008;21:507–11. [PubMed] [Google Scholar]
- 129.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–65. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 130.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, et al. SIRT1 Regulates Hepatocyte Lipid Metabolism through Activating AMP-activated Protein Kinase. J Biol Chem. 2008;283:20015–26. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization and activity of LKB1; possible role in AMP-activated protein kinase activation. J Biol Chem. 2008 doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Habinowski SA, Witters LA. The effects of AICAR on adipocyte differentiation of 3T3-L1 cells. Biochem Biophys Res Commun. 2001;286:852–6. doi: 10.1006/bbrc.2001.5484. [DOI] [PubMed] [Google Scholar]
- 133.Sullivan JE, Brocklehurst KJ, Marley AE, Carey F, Carling D, Beri RK. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994;353:33–6. doi: 10.1016/0014-5793(94)01006-4. [DOI] [PubMed] [Google Scholar]
- 134.Misra P. AMP activated protein kinase: a next generation target for total metabolic control. Expert Opin Ther Targets. 2008;12:91–100. doi: 10.1517/14728222.12.1.91. [DOI] [PubMed] [Google Scholar]
- 135.Maechler P. Novel regulation of insulin secretion: the role of mitochondria. Curr Opin Investig Drugs. 2003;4:1166–72. [PubMed] [Google Scholar]
- 136.Rutter GA. Nutrient-secretion coupling in the pancreatic islet beta-cell: recent advances. Mol Aspects Med. 2001;22:247–84. doi: 10.1016/s0098-2997(01)00013-9. [DOI] [PubMed] [Google Scholar]
- 137.Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–17. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 138.Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Redinger RN. The physiology of adiposity. J Ky Med Assoc. 2008;106:53–62. [PubMed] [Google Scholar]
- 140.Retnakaran R, Youn BS, Liu Y, Hanley AJ, Lee NS, Park JW, et al. Correlation of circulating full-length visfatin (PBEF/Nampt) with metabolic parameters in subjects with and without diabetes: a cross-sectional study. Clin Endocrinol (Oxf) 2008 doi: 10.1111/j.1365-2265.2008.03264.x. [DOI] [PubMed] [Google Scholar]
- 141.Sandeep S, Velmurugan K, Deepa R, Mohan V. Serum visfatin in relation to visceral fat, obesity, and type 2 diabetes mellitus in Asian Indians. Metabolism. 2007;56:565–70. doi: 10.1016/j.metabol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 142.Kong AP, Chan NN, Chan JC. The role of adipocytokines and neurohormonal dysregulation in metabolic syndrome. Curr Diabetes Rev. 2006;2:397–407. doi: 10.2174/1573399810602040397. [DOI] [PubMed] [Google Scholar]
- 143.Tilg H, Moschen AR. Role of adiponectin and PBEF/visfatin as regulators of inflammation: involvement in obesity-associated diseases. Clin Sci (Lond) 2008;114:275–88. doi: 10.1042/CS20070196. [DOI] [PubMed] [Google Scholar]
- 144.Goralski KB, Sinal CJ. Type 2 diabetes and cardiovascular disease: getting to the fat of the matter. Can J Physiol Pharmacol. 2007;85:113–32. doi: 10.1139/y06-092. [DOI] [PubMed] [Google Scholar]
- 145.Sethi JK. Is PBEF/visfatin/Nampt an authentic adipokine relevant to the metabolic syndrome? Curr Hypertens Rep. 2007;9:33–8. doi: 10.1007/s11906-007-0007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Haider DG, S G, Kapiotis S, Maier C, Luger A, Wolzt M. The release of the adipokine visfatin is regulated by glucose and insulin. Diabetologia. 2006;49:1909–14. doi: 10.1007/s00125-006-0303-7. [DOI] [PubMed] [Google Scholar]
- 147.da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J. 2003;371:761–74. doi: 10.1042/BJ20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Salt IP, Johnson G, Ashcroft SJ, Hardie DG. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem J. 1998;335(Pt 3):533–9. doi: 10.1042/bj3350533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leclerc I, Woltersdorf WW, da Silva Xavier G, Rowe RL, Cross SE, Korbutt GS, et al. Metformin, but not leptin, regulates AMP-activated protein kinase in pancreatic islets: impact on glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2004;286:E1023–31. doi: 10.1152/ajpendo.00532.2003. [DOI] [PubMed] [Google Scholar]
- 150.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, et al. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–19. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 151.Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–81. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 153.Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004;314:580–5. doi: 10.1016/j.bbrc.2003.12.120. [DOI] [PubMed] [Google Scholar]
- 154.Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996;81:4059–67. doi: 10.1210/jcem.81.11.8923861. [DOI] [PubMed] [Google Scholar]
- 155.Le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol. 2007;19:628–33. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Le Grand F, Rudnicki M. Satellite and stem cells in muscle growth and repair. Development. 2007;134:3953–7. doi: 10.1242/dev.005934. [DOI] [PubMed] [Google Scholar]
- 157.McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, et al. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gopinath SD, Rando TA. Stem Cell Review Series: Aging of the skeletal muscle stem cell niche. Aging Cell. 2008 doi: 10.1111/j.1474-9726.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- 159.Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab. 2007;292:E151–7. doi: 10.1152/ajpendo.00278.2006. [DOI] [PubMed] [Google Scholar]
- 160.Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005;4:407–10. doi: 10.4161/cc.4.3.1518. [DOI] [PubMed] [Google Scholar]