Abstract
The aims of this study were to determine (i) the effect of passive hyperthermia on motor drive and cognitive function, and (ii) whether head cooling can limit the hyperthermia-induced alterations. Sixteen subjects were randomly exposed for 2 h to three different conditions: control (Con, 20°C), hot (Hot, 50°C) and hot head cool (HHC – where cold packs were applied to the head under Hot conditions). Three cognitive tests measuring attention and two measuring memory were performed. Neuromuscular testing included electrically evoked muscle action potentials (M-waves) and reflex waves (H-reflex) at rest and during brief (4–5 s) and sustained (120 s) maximal voluntary contractions (MVC) of the plantar flexors. All the tests were performed in the environmental room. During brief MVC, torque was significantly lower in both Hot and HHC as compared to Con (P < 0.05). The decrease in muscle activation was significant in Hot (P < 0.05) but not in HBC (P= 0.07). This was accompanied by peripheral failures in the transmission of the neural drive at both spinal (significant decrements in H-reflexes and V-waves, P < 0.05) and neuromuscular junction (significant decrements in M-waves, P < 0.05) levels. During sustained MVC, muscle activation was further depressed (P < 0.05) without any concomitant failures in M-waves, suggesting neural activation adjustments occurring probably at the supraspinal level. Cerebral perturbations were confirmed by significant decrements in both memory tests in Hot as compared with Con (P < 0.05) but not in simple tests (attention tests) that were not affected by hyperthermia. The decrement in memory capacity suggested the existence of frontal lobe activity impairments. Thus, HHC preserved memory capacity but not the visual memory.
Previous studies reported central fatigue following completion of an exercise in a warm environment (Nybo & Nielsen, 2001). Central fatigue has also been reported after completing exercise in a neutral environment (Gandevia et al. 1996; Taylor et al. 2006; Racinais et al. 2007a). Consequently, passive hot exposure represents a useful model to avoid the exercise-related side-effects when investigating hyperthermia. Decrements in cognitive function (Cian et al. 2001), voluntary force production (Morrison et al. 2004; Thomas et al. 2006) and voluntary activation (VA) (Morrison et al. 2004; Thomas et al. 2006) have been observed after passive hyperthermia. This failure in VA partly occurs in cortical areas at or above motor cortex level during a 120 s contraction (Todd et al. 2005). Cognitive function alterations induced by hyperthermia (Cian et al. 2001; Hocking et al. 2001; McMorris et al. 2006) are another indicator of cortical perturbation. The effect of this perturbation on both neural drive and cognitive function are task related. Previous studies showed that complex cognitive tasks were impaired by hyperthermia (Cian et al. 2001; McMorris et al. 2006) whereas simple and short-duration tasks were not (Amos et al. 2000). Following the same pattern, supraspinal perturbations were observed to alter neural drive during a sustained MVC (Todd et al. 2005) but the origin of the decrement in VA is still unknown for brief MVC. The contribution of other potential sites (e.g. motoneural and sarcolemmal excitability) still needs to be addressed.
One of the major sites for modulation in the motor drive is the spinal cord. In neutral environments, spinal modulation of the neural drive has been observed after an isometric contraction maintained until exhaustion (Duchateau & Hainaut, 1993; Duchateau et al. 2002) or after sub-maximal running exercise (Racinais et al. 2007b). As this modulation was partly related to a presynaptic inhibition mediated by group III and IV afferents (Bigland-Ritchie et al. 1986; Woods et al. 1987; Garland & McComas, 1990; Garland, 1991; Duchateau et al. 2002; Avela et al. 2006) that are temperature sensitive, a spinal modulation of the neural drive is likely to occur in a hot environment. Therefore, the first aim of this study was to determine the effect of passive hyperthermia on cognitive and neuromuscular functions for both simple/short and complex/long tasks. We hypothesized that simple cognitive tasks should not be impaired by hyperthermia whereas brief MVC would decrease in relation to perturbations occurring below cortical area.
Furthermore, core temperature rather than local thermal afferent input from the skin was suggested to be responsible for the alteration in neural drive (Thomas et al. 2006). Animal models showed that rats stop exercising in a hot environment at the same abdominal and cerebral temperatures regardless of the modification made to their initial temperature (Fuller et al. 1998). In addition, when increasing the cerebral temperature close to 42°C, a goat reduces its velocity or refuses to move (Caputa et al. 1986). In the human brain, heat release is inadequate during prolonged exercise with hyperthermia, leading to higher brain temperature than core temperature (Nybo et al. 2002). Combined, these studies suggest that cerebral temperature could be the key factor leading to an alteration in animal activities. Following this hypothesis, cooling the head was showed to improve thermal comfort in humans with (Simmons et al. 2008) or without (Mündel et al. 2006) partly protecting core temperature during passive heating. However, the functional consequences of applying cool packs to the head (head cooling) on both cognitive and neurophysiological functions were never investigated. Therefore, the second aim of this study was to determine how cooling the head during whole-body hyperthermia could reduce the alteration in both neural drive and cognitive function.
Methods
Ethical approval
The project was approved by the ASPETAR Human Research Ethics Committee (including 2 scientists, 2 medical doctors, 1 psychologist, 1 lawyer, 2 members of humanitarian associations, 1 religious representative and 1 administrator). The procedures complied with the Declaration of Helsinki regarding human experimentation. Written informed consent was obtained from all the participants before the beginning of the testing.
Subjects
Sixteen volunteers (11 males and 5 females, 31 ± 1 years, 73 ± 3 kg and 175 ± 3 cm for age, weight and height, respectively) participated in the study. None of the subjects suffered from muscle soreness or ankle injuries at the time of the experiment. Subjects were asked to avoid all vigorous activity for the 24 h preceding the test.
General procedure
Following an initial familiarization session, subjects randomly participated in three experimental trials with 4 to 7 days of recovery between them. The tests (acclimation, cognitive and neuromuscular testing) were performed in an environmental chamber (Tescor, Warminster, PA, USA), at the same time of day, with subjects wearing shorts and t-shirts.
Familiarization trial
The subjects were introduced to and familiarized with the experimental procedures for both the cognitive and neuromuscular testing. The software used for the battery of cognitive testing (see below) provided a familiarization procedure for each test. In addition, subjects performed the complete procedure of the subsequent trial. Thereafter, they performed maximal voluntary contractions (MVC) of the plantar flexors until they felt accustomed to the equipment; the coefficient of variation in three successive trials was less than 5%. This session was also used to accustom the subjects to the electrical stimulation of the tibial nerve. Following this trial, 16 of the initial 19 subjects volunteered to participate in the experimental sessions.
Control trial (Con)
After 20 min of rest (for electrode placement, verification of the signals and stabilization of the values), subjects entered the environmental chamber and walked on a treadmill for 10–15 min at 3–5 km h−1 depending on their fitness level. This procedure augmented heat production without inducing exercise fatigue. Subjects then rested in a seated position for 45 min before starting the cognitive and neuromuscular testing session (described below) (Fig. 1). The room was set at 20°C and 40% relative humidity and the average wet bulb globe temperature (WBGT) recorded (QUESTempo36, Quest Technologies, Oconomowoc, WI, USA) during the session was 22.8 ± 0.6°C.
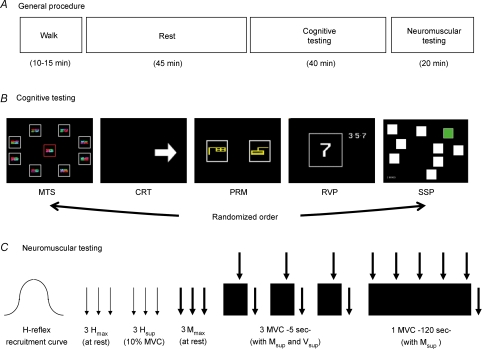
Figure 1.
A, general procedure. B, cognitive testing: the 5 cognitive tests were performed randomly within subjects (MTS, match to sample visual search; CRT, choice reaction time; PRM, pattern recognition memory; RVP, rapid visual information processing; SSP, spatial span). C, neuromuscular testing: thin arrows: stimulation at Hmax intensity; thick arrows: stimulation at Mmax intensity; black rectangles: maximal voluntary contraction (MVC).
Hot trial (Hot)
The procedure was the same as Con, but with the room set at 50°C and 50% relative humidity. The average WBGT recording during the session was 43.1 ± 0.8°C.
Hot head cool trial (HHC)
This was performed under the same environmental conditions as Hot (mean WBGT: 44.9 ± 0.8°C) but with three cool packs applied to the head and one to the neck. The cool packs (Nexcare, 3M, St Paul, MN, USA) were frozen (−14°C), applied with a protective layer (between the skin and the cool pack) and changed regularly (mean time: ∼20 min).
Temperature recording
Core temperature (Tcore) and skin temperatures were monitored using the VitalSense system (precision 0.01°C, Mini Mitter, Respironics, Herrsching, Germany). A wireless Jonah ingestible thermometer pill, swallowed several hours before the testing session, measured Tcore. Skin temperatures of the gastrocnemius medialis of the right leg (Tleg) and the forehead (Thead) were monitored by XTP wireless dermal adhesive temperature patches. Both sensors sent data by telemetry to the same recording monitor every 60 s. Tympanic temperature (Ttymp) was also recorded before and after each test session with an infrared thermometer (MP7 Qiuick, Medel, S. Polo di Torrile, Italy). The temperatures recorded before and after the testing sessions were averaged to provide the temperature of the session.
Cognitive testing session
Subjects performed five different cognitive tests using Cantab software (CANTABeclipse, Cambridge Cognition, Cambridge, UK) and hardware (tactile screen and touch pad) in the environmental chamber under constant noise and lighting conditions. These tests are based on standard cognitive tests (description below) that are regularly used in neuropsychological assessment (e.g. Joyce et al. 1996; Swainson et al. 2001). An example of each the following tests is displayed in Fig. 1. The sequence of the test was counterbalanced within the subject but identical for the three sessions of a given subject.
Attention tests
The match to sample (MTS) visual search tests the ability to match visual stimuli and measures reaction time (in ms). A sample stimulus was displayed in the middle of the screen. After a brief delay, one to eight similar patterns were shown around the sample. The subject had to touch the one matching the sample. The choice reaction time (CRT) measures speed of response (in ms) in a simple two choice protocol. Subjects had to press as fast as possible the button of the press pad that corresponded to the direction of an arrow displayed on the screen. The rapid visual information processing (RVP) assesses visual sustained attention. Digits from 2 to 9 were displayed in the middle of the screen at a rate of 100 digits per minutes. Subjects were instructed to detect target sequences of digits (i.e. 2–4–6, 3–5–7, 4–6–8) by pressing the press pad and the number of missed target sequences was recorded.
Working and visual memory tests
The spatial span (SSP) assesses working memory capacity. Subjects had to remember increasing number of squares (up to 9) illuminated one by one in a variable sequence and then to touch them in the same order, on the screen. The maximum number of squares successfully remembered was recorded. The pattern recognition memory (PRM) tests visual recognition memory. After looking at a series of 12 visual patterns, subjects had to choose between the one that they had already seen and a new one. The percentage of correct answers was recorded.
Neuromuscular testing session
Subjects were seated, head motionless, with ankle and knee angulations of, respectively, 90 deg and 100 deg, and the foot securely strapped to a dynamometric pedal (Captels, St Mathieu de Treviers, France).
Evoked potentials
The tibial nerve was stimulated by a cathode placed in the popliteal cavity and an anode distal to the patella. Electrical stimulations (max voltage 400 V, rectangular pulse of 0.2 ms) were delivered by a high-voltage stimulator (Digitimer DS7AH, Digitimer, Hertfordshire, UK). The amperage was adjusted for each subject by a progressive increase (10 mA increment) until a plateau in both twitch mechanical response (peak twitch, Pt) and electrophysiological response (M-wave amplitudes (Mmax)). With increasing stimulation intensity, the H-reflex response initially increased progressively before decreasing and then disappearing. The intensity needed to obtain maximal amplitude in the reflex wave (Hmax) was adjusted by 1 mA and checked before each testing session. During a MVC, stimulation at Mmax intensity induced a superimposed action potential (Msup) followed an electrophysiological variant of the H-reflex called V-wave (Vsup) (Upton et al. 1971; Aagaard et al. 2002; Racinais et al. 2007b).
The amplitude of the electrically evoked action potentials were recorded on the soleus by integral dry reusable electrodes with an inter-electrode distance of 20 mm (Biometrics SX230, Gwent, UK) and a reference electrode on the wrist. Skin was shaved, abraded and washed for low impedance. Signals were recorded at a sampling frequency of 5000 Hz using Biometrics hardware (Biometrics DataLOG, Gwent, UK) and dedicated software.
Calculations
The Pt is considered as an index of the contractile properties and Mmax amplitude represents an index of sarcolemmal excitability. M-wave amplitude was also used to normalize the amplitude of the reflex waves recorded to ensure that any changes in the evoked Hmax and Vsup amplitudes were not due to changes at the muscle fibre membrane or neuromuscular junction. The Hmax/Mmax ratio represents a global index of the spinal modulation depending on motoneurone excitability as well as Renshaw cell and presynaptic inhibition, even if the latter are of minor effect during testing at rest. The Vsup/Msup ratio is also partly dependent on the collision in antidromically activated axons but, as the underpinning V-wave mechanisms are still unclear, these data were only used to confirm the H-reflex data (Aagaard et al. 2002; Racinais et al. 2007b). In addition, the Hmax was also recorded during a submaximal contraction performed at a constant intensity (determined during the familiarization session to correspond at 10% of MVC) to ensure a constant level of background muscle activity (Zehr, 2002). The latencies of the electrically evoked action potentials were calculated from the stimulation artifact to the peak of the wave. The ratio of the amplitude of a superimposed twitch torque over the amplitude of a twitch evoked at rest 4 s after the MVC (potentiated twitch) was used to assess the level of voluntary activation (VA) as follow: VA (%) = (1 – Superimposed twitch/Potentiated twitch) × 100.
Protocol
The neuromuscular tests are described in Fig. 1. All the testing procedure began with the adjustment of the stimulation intensity required to induce a maximal H-reflex in soleus muscle (simplified procedure based on the knowledge of the values from the familiarization trial). Afterwards, three H-reflexes (interspaced by 10 s) were elicited from both the relaxed muscle (Hmax) and during a constant (10% of the MVC of the familiarization trial) muscle contraction (Hsup). Three Mmax interspaced by 8 s were evoked from the relaxed muscle. Thereafter, subjects were instructed and verbally encouraged to perform three brief MVC (4–5 s) of the plantar flexor muscles with at least 1 min rest period between contractions. A superimposed stimulus (Mmax intensity) was evoked in order to obtain the M-wave (Msup) and V-wave (Vsup). For all of the MVC or evoked waves and twitches, the values of three trials were averaged for subsequent analysis. Afterward, one sustained MVC (120 s) was performed with superimposed stimuli at 3, 30, 60, 90 and 120 s as well as 4 s post-contraction.
Statistical analysis
The effect of the environmental condition was analysed for each variable by a one-way analysis of variance (ANOVA) for repeated measures (3 conditions). For the data repeatedly recorded during the MVC of 120 s, a 2-way ANOVA for repeated measures (5 times, 3 conditions) was used. Pair-wise comparisons were applied post hoc to further investigate the effect of each condition. Statistical analyses were performed with Systat software (Systat, Evanston, IL, USA). Data are reported as mean ±s.e.m. and the level of statistical significance was set at P≤ 0.05.
Results
Temperature
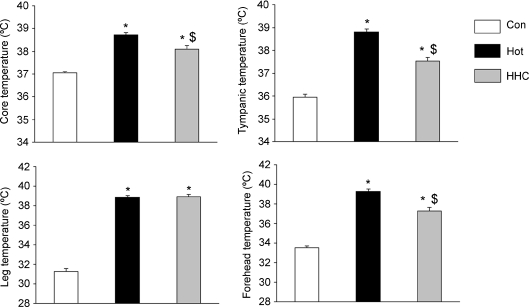
The average body temperatures were significantly higher (P < 0.001) during both Hot and HHC testing sessions than in Con (Fig. 2). Furthermore, cooling the head and neck of the subjects reduced Tcore, Ttymp and Thead by 0.6°C, 1.3°C and 2.0°C, respectively, compared to Hot (F1,15 > 19.32, P < 0.001 for all), without any modification in Tleg (+0.1°C, F1,15= 0.14, N.S.).
Figure 2. All the body temperatures were increased in hot environment (Hot) in comparison to neutral environment (Con).
Cooling the head in a hot environment (HHC) reduced the temperature at some sites but not at the peripherally and with values still higher than Con. *higher than Con, P < 0.05: $lower than Hot, P < 0.05.
Due to hydration ad libidum, body weight was not modified by either heat exposure whatever the condition (values post-exposure: 72.9 ± 3, 73.1 ± 3 and 73.1 ± 3 kg for Con, Hot and HHC, respectively, F2,30= 1.53, N.S.).
Neuromuscular testing
MVC 5 s
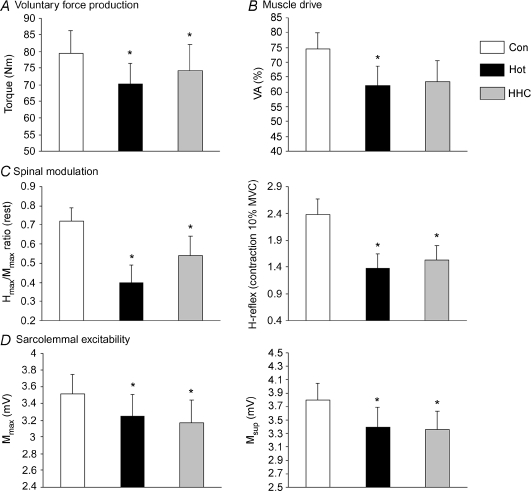
The torque production was significantly dependent on the environmental condition (Fig. 3A, F2,30= 4.97, P < 0.02) with lower values in both Hot (−10.9%) and HHC (−8.3%) than Con (F1,15 > 5.23, P < 0.05 for both), but without difference between Hot and HHC (F1,15= 1.51, N.S.). These decrements were associated with significant variations in VA (Fig. 3B, F2,30= 3.78, P < 0.05) with Hot values significantly lower as compared with Con (−11.9%, F1,15= 5.20, P < 0.05) whereas HHC values did not reach significance (−13.7%, F1,15= 3.73, P= 0.07). Muscle contractility did not differ between conditions (Pt: 20.4 ± 1, 18.8 ± 1 and 19.8 ± 1 Nm for Con, Hot and HHC, respectively, F2,30= 1.68, N.S.).
Figure 3.
A, voluntary force production was reduced in hot environment (Hot) in comparison with neutral environment (Con), even if the head was maintained cooler (HHC). This decrement can be linked to alteration in muscle drive (B), spinal modulation (C) and sarcolemmal excitability (D). *P < 0.05.
Electrically evoked potentials
Due to the stimulation intensity adjustment, the small M-waves associated with the H-reflexes were not dependent on the trial (F2,24 < 2.54, N.S., for both Hmax and Hsup). Amplitudes of the electrically evoked reflex waves (Table 1, Fig. 3C) were significantly reduced by hyperthermia (F2,28 > 3.73, P < 0.05 for Hmax, Hsup, Vsup, Hmax/Mmax and Vsup/Msup ratio).
Table 1.
Amplitudes and latencies of the electrically evoked action potentials during the control condition (Con), in a hot environment (Hot) and in hot with the head cooled (HHC)
| Amplitude (mV) | Latency (ms) | |||||
|---|---|---|---|---|---|---|
| Con | Hot | HHC | Con | Hot | HHC | |
| At rest | ||||||
| 3.52 | 3.25* | 3.17* | Mmax | 14.1 | 12.9* | 13.1* |
| 0.23 | 0.26 | 0.27 | 0.4 | 0.4 | 0.4 | |
| 2.38 | 1.51* | 1.77* | Hmax | 38.7 | 36.6* | 37.1* |
| 0.32 | 0.31 | 0.33 | 0.7 | 0.7 | 0.8 | |
| 0.72 | 0.40* | 0.54* | Hmax/Mmax | |||
| 0.07 | 0.09 | 0.10 | (a.u) | |||
| Submaximal contraction (10% of MVC) | ||||||
| 2.38 | 1.37* | 1.53* | Hsup | 38.1 | 35.8* | 36.6* |
| 0.29 | 0.28 | 0.27 | 0.7 | 0.7 | 0.8 | |
| Maximal contraction | ||||||
| 3.80 | 3.40* | 3.36* | Msup | 11.4 | 10.5* | 10.0 |
| 0.25 | 0.29 | 0.27 | 0.3 | 0.3 | 0.7 | |
| 1.05 | 0.78* | 0.88 | Vsup | 36.9 | 34.4* | 35.2*$ |
| 0.2 | 0.19 | 0.19 | 0.6 | 0.6 | 0.8 | |
| 0.37 | 0.21* | 0.25* | Vsup/Msup | |||
| 0.08 | 0.04 | 0.06 | (a.u) | |||
Lower than Con
higher than Hot, P < 0.05. Data is mean ± S.E.M.
In addition, our results displayed a drop in the amplitude of the M-waves (Table 1, Fig. 3D) from Con in both Hot and HHC (F1,15 > 5.31, P < 0.05 for both Mmax and Msup).
The latency of all electrically evoked action potentials (Mmax, Hmax, Msup and Vsup, Table 1) decreased significantly in Hot (Con versus Hot: F1,15 > 41, P < 0.001) without any effect of head cooling (Hot versus HHC: F1,15 < 2.01, N.S.), except for Vsup latency, which was slower in HHC than in Hot (F1,15= 8.9, P < 0.01) but still faster than Con (F1,15= 27.4, P < 0.001).
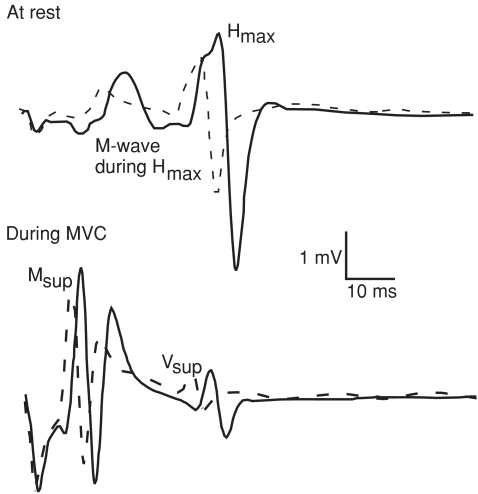
Recordings of electrically evoked potentials in a representative subject are displayed in Fig. 4.
Figure 4.
Example of electrically induced waves recorded at rest (top graph) and during MVC (bottom graph) in one subject in both neutral (Con, continuous line) and hot (Hot, dashed line) environment.
MVC 120 s
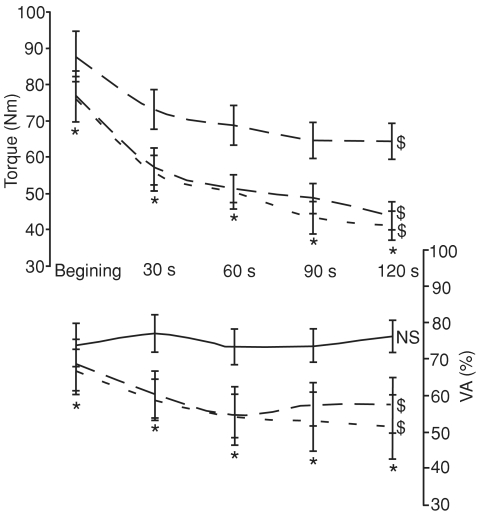
Torque production (Fig. 5) decreased across the 120 s of contraction (F4,60= 48.2, P < 0.001) and was lower in both Hot and HHC than Con throughout the contraction (F2,30= 24.2, P < 0.001). Moreover, torque data displayed an interaction effect within time and condition due to a larger decrement rate in both Hot and HHC than in Con (F8,120= 2.42, P < 0.02, Fig. 5).
Figure 5.
Torque significantly decreased across the 120 s sustained MVC in all conditions (Control, continuous line; Hot, dashed line; hot head cool, dotted line). $Significant decrease throughout the contraction, P < 0.05. The higher decrement in torque observed in Hot and HHC is linked to a decrement in the level of voluntary activation (VA) in these conditions. *Hot and HHC lower than Con, P < 0.05. N.S., not significant.
The level of VA (Fig. 5) followed a similar pattern of evolution to torque with an effect of the environmental condition (F2,26= 11.5, P < 0.001), an effect of the contraction time (F4,52= 2.73, P < 0.05) and an interaction effect (F8,104= 2.16, P < 0.05, Fig. 5). The level of VA remained stable across the 120 s of contraction in Con (F4,52= 0.37, N.S.) but significantly decreased during the contractions performed both in Hot (F4,52= 3.13, P < 0.05) and HHC (F4,52= 3.25, P < 0.05). The action potential associated with the superimposed twitch (Msup) was depressed in both Hot (average: 3.56 ± 0.12 mV, F1,15= 24.03, P < 0.001) and HHC (average: 3.63 ± 0.11 mV, F1,15= 61.70, P < 0.001) when compared to Con (average: 4.36 ± 0.08 mV), without any effect of the contraction time (F8,120= 0.747, N.S.). In parallel, Msup slightly increased (F4,60= 2.84, P < 0.05) from the beginning (3.7 ± 0.2 mV) to the end (3.9 ± 0.1 mV) of the sustained contraction, independently of the environmental condition (F8,120= 0.747, N.S.).
Cognitive testing
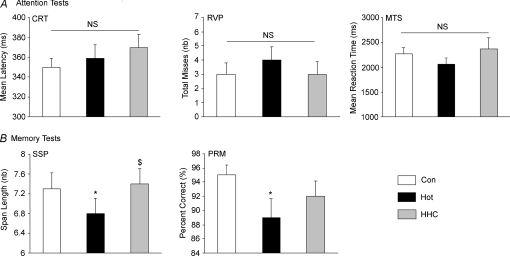
No significant between trial differences were recorded in the three attention tests (CRT, MTS and RVP, F2,30 < 1.57, N.S., Fig. 6A). However, the two memory tests displayed an effect of the environmental condition (SSP and PRM, F2,30 > 4.16, P < 0.05, Fig. 6B). The longest successfully recalled pattern sequence of the SSP was significantly lower in the Hot than both Con (F1,15= 6.0, P < 0.05) and HHC (F1,15= 9.57, P < 0.01). The percentage of correct answers in the PRM test was lower in Con than in Hot (F1,15= 6.91, P < 0.02) and in HHC (F1,15= 4.46, P= 0.05).
Figure 6.
A, attention test. CRT, choice reaction time; RVP, rapid visual information processing; MTS, match to sample visual search. B, memory test. SSP, spatial span; PRM, pattern recognition memory. *Lower than Con, P≤ 0.05; $higher than Hot, P < 0.05; N.S., not significant.
Discussion
In line with previous reports (Morrison et al. 2004; Thomas et al. 2006), we observed an alteration in voluntary muscle force production following passive hyperthermia (Fig. 3A). For the first time, our results showed that this alteration is not related to muscle contractility alterations but is accompanied by perturbations in peripheral transmission of the neural drive (Fig. 3C and D) as well as significant decreases in working and visual memory (Fig. 6). In addition, data showed that VA further decrease during a prolonged MVC in hot environment only (Fig. 5).
Neuromuscular junction failures
The decrement in VA (Fig. 3B) can be linked to a modification in any of the stages from the cortical activity to sarcolemma depolarization. Previous studies hypothesized that this failure could represent a conscious or unconscious anticipatory decrement in voluntary motor drive to avoid further heat production and protect the body (and the brain itself) from an additional rise in temperature (Marino et al. 2004; Tucker et al. 2004, 2006). However, the theory of an anticipatory decrement in exercise intensity cannot be transposed to passively induced hyperthermia. Following passive heating, our data showed that the decrement in VA is partly linked to peripheral alterations in the transmission of the motor drive. Our results displayed a significant decrease in both Mmax and Msup with hyperthermia (Table 1, Fig. 4) showing that a given amount of (electrically induced) neural drive failed to induce an equivalent sarcolemmal action potential in hyperthermia. This decrement can be explained by failures in the synaptic transmission at the neuromuscular junction level. In vitro studies confirm this hypothesis by showing modification in synaptic transmission at high temperature (Kelty et al. 2002). The stimulation of a nerve causes the release of a variable number of quanta per impulse during a train of stimuli, but if postsynaptic quantal units go undetected following a stimulus, this is termed a failure (Karunanithi et al. 1999). It was previously observed that at 22°C all the synapses produced one or more quantal events for each nerve impulse without any failure but as temperature increased, the amplitude of the response declined and failures became evident until transmission completely failed when the nerve temperature reached 35°C (Karunanithi et al. 1999). However, these observations were done in vitro in Drosophila synapses with a range of temperatures lower than those recorded in vivo in hyperthermic humans (close to 39°C for both Tcore and Tleg in our experiment). Our data add a functional perspective to these observations by showing that whole-body hyperthermia also affects the synaptic transmission in healthy humans. This supports the previously observed decrement in Mmax at rest with localized warming of the leg (Dewhurst et al. 2005), which was attributed to a shortening of the depolarization time when temperature increased, consequently allowing less Na+ to enter the cell (Rutkove, 2001).
Spinal modulations
In parallel, as our results showed a significant drop in VA (Fig. 3B) estimated by twitch interpolation, the extra force evoked by the superimposed stimulus on the motor nerve during MVC attests to the possibility for more α-motoneurones to discharge and/or additional opportunity for the sarcolemma to depolarize. That suggests that the peripheral failure in synaptic transmission observed at the sarcolemmal level could also be present at the spinal level. In this study we used electrically evoked reflex waves to estimate the effect of hyperthermia on the spinal loop efficiency. Our results showed a decrement in the amplitude of the reflex waves whether expressed absolutely or normalized by the change occurring at the sarcolemmal level both in Hot and HHC (Table 1). To the best of our knowledge, the only study investigating the effect of passive warming on H-reflex failed to observe any modification in this parameter (Dewhurst et al. 2005). However, this study was only based on a local warming of the leg (Dewhurst et al. 2005), that induced a drop in M-wave (as observed in this study) but had no influence on spinal cord synapses. Moreover, H-reflex decrements were confirmed by a decrease in V-waves (an electrophysiological variant of the H-reflex, Table 1, Fig. 4). These decrements in electrically evoked reflex waves can be linked to both the inhibition/control loop acting presynaptically and alterations in the excitability of the postsynaptic element (i.e. the motoneurone). Presynaptically, the decline in transmission from the Ia afferent stimulation to α-motoneurone excitation could be a consequence of a presynaptic inhibition mediated by group III and IV afferents (Bigland-Ritchie et al. 1986; Woods et al. 1987; Garland & McComas, 1990; Garland, 1991; Duchateau et al. 2002; Avela et al. 2006). The duration of this presynaptic inhibition will depend on whether the input that is producing the inhibition is ongoing or has ceased. As these afferents are sensitive to temperature, which was an ongoing factor in this experiment, input of group III and IV muscle afferents could represent a valuable explanation for the decrement in spinal reflexes observed in Hot and HHC (Figs 3C and 4). However, previous studies have reported that maintaining the muscle in an ischaemic state did not affect the altered responses to transcranial stimulation (Gandevia et al. 1996; Taylor et al. 2000; Andersen et al. 2003) suggesting that group III and IV muscle afferents do not directly inhibit motoneurones but act upstream of the motor cortex to impair voluntary descending drive (Taylor et al. 2006). Furthermore, in light of the current data showing a significant depression in the amplitude of the electrically evoked action potentials (M-waves, Table 1, Fig. 4), we suggest that intrinsic hyperthermia-induced alteration of the spinal synapses and the α-motoneurones have to be considered.
Supraspinal alterations
As our data suggest significant alterations in the synaptic transmission of the neural influx at both peripheral and spinal level, it questions the possibility of similar alterations at the supraspinal level. In this study, cognitive function was used as an index of alteration occurring at the cortical level. Literature regarding the effect of hyperthermia on cognitive function is equivocal reporting either no influence (Amos et al. 2000) or a detrimental effect (Hocking et al. 2001). In our study, simple tests (RVP, MTS and CRT, Fig. 6A) were unaffected by passive hyperthermia, while significant decrements in both working memory capacity (SSP test) and visual recognition (PRM test) were observed (Fig. 6B). An absence of alteration in reaction time in a hot environment was previously linked to an offset within the negative (premotor reaction time) and beneficial (nerve conduction velocity) effect of heat exposure on the different component parts of reaction time (Aird et al. 1983). Our data showing a significant improvement in nerve conduction velocity in both Hot and HHC (Table 1, Fig. 4) could explain why CRT wasn't altered in these conditions whereas some other tests were. The complexity of the task seems also to be a factor when assessing the effect of hyperthermia. Unlike the speed and accuracy of simple cognitive functions (Amos et al. 2000), complex tasks (i.e. working memory, information retention and visual speed of information processing, central executive tasks) have been observed to be altered by hyperthermia (Cian et al. 2001; McMorris et al. 2006). Our data from standardized conditions (passive hyperthermia, euhydrated subjects) showed an alteration in Hot of the complex tasks only, confirming that the detrimental effects of hyperthermia are task complexity dependant. The SSP and PRM tests were previously demonstrated to be sensitive to chronic fatigue (Joyce et al. 1996) and disorders such as mild to moderate Alzheimer's disease (Swainson et al. 2001), respectively. Our data additional show that these indexes of working memory can also be altered in Hot (Fig. 6B), possibly leading to functional implications for people working in hot environments. For example, safety-related behaviour of workers has been observed to be detrimentally affected by thermal conditions (Ramsey et al. 1983) but returning to a neutral environment can restore central executive tasks (McMorris et al. 2006).
Cooling the head
Equilibrium between heat release and production in the human brain is maintained during cycling exercise in a neutral environment (Nybo et al. 2002). This balance is lost during exercise in a hot environment with an increased brain heat storage increasing brain temperature to a greater extend than core temperature (Nybo et al. 2002). In an attempt to find a solution to preserve neural function in a hot environment, this study investigated the efficiency of applying cool packs locally to the head (HHC). This method was previously demonstrated to be more efficient than cool air breathing to decrease heat strain (Desruelle & Candas, 2000). Our results showed that this selective cooling attenuated the increase in forehead as well as tympanic temperature (Fig. 2) which was previously demonstrated to be the best indicator of brain temperature (Mariak et al. 2003). Cooling the head was shown to improve subjective comfort (Mundel et al. 2006; Simmons et al. 2008) but the repercussion of this improvement on cognitive abilities was still unknown. Our data show that HHC blunted the hyperthermia-induced alteration in working memory capacity (SSP test) and allowed this parameter to return to control values (Fig. 6B). However, the visual recognition (PRM test) was also altered by hyperthermia but failed to be significantly protected by HHC. This difference in the effect of HHC can be related to the different areas of the brain involved in these two functions. The PRM is sensitive to dysfunction in temporal (Owen et al. 1995) and medial temporal areas of the brain (Galloway et al. 1992), whereas SSP is a computerized version of the Corsi Blocks task, sensitive to frontal lobe dysfunction (Morris et al. 1988; Malhotra et al. 2005). The significant decrease in forehead temperature with HHC (Fig. 2) can explain its protective effect on this particular brain area. Even though a potential effect of the slightly lower core temperature in HHC than in Hot cannot be ruled out, the decrements in Ttymp (−1.3°C) and Thead (−2.0°C) were larger in amplitude than Tcore (−0.6°C). This suggests an effect of head temperature on cognitive function and gives a functional significance to previous data observing alterations in the electroencephalographic activity of the frontal cortex with active hyperthermia-induced fatigue (Nielsen et al. 2001).
While our results showed that HHC can significantly protect some cognitive functions, we did not observe any significant beneficial effect of HHC on the neuromuscular function. The observation that maintaining the leg at a neutral temperature failed to protect VA from decreasing (Thomas et al. 2006) supports an earlier hypothesis that core rather than skin temperature was the determining factor in VA alteration (Morrison et al. 2004). Animal studies focusing on cerebral temperature have shown that goats reduce their velocity or refuse to move when cerebral temperature was passively increased (Caputa et al. 1986). Taken together, these observations suggest that brain temperature could regulate the level of VA. However, the finding that HHC significantly reduced tympanic and forehead temperature but did not modify the leg temperature (Fig. 2) nor the muscle activation (Fig. 3B) confirms that muscle activity decrement is also linked to peripheral alterations and not only to supraspinal failure. It has been suggested that the supraspinal limitation to muscle force production with hyperthermia could increase with the contraction duration. Following exhaustive exercise in a hot environment, VA can remain unaltered for a contraction lasting a few seconds but be impaired if the contraction is prolonged (> 10 s) (Nybo & Nielsen, 2001). This suggests that the capacity of the central nervous system (CNS) to maximally activate the muscle after hyperthermia may be altered differently if the outputs have to be continuously maintained (Nybo & Nielsen, 2001; Martin et al. 2005). However, comparable data were observed in a neutral environment with an earlier and larger central activation deficit during a continuous elbow extension task than during the same task performed intermittently (Bilodeau, 2006). This makes it difficult to conclude that the CNS failure observed in these previous studies was linked to the temperature itself and not to the exercise performed to induce hyperthermia. With a 120 s MVC performed during passive hyperthermia, our results showed that Msup was reduced by hyperthermia from the beginning of the contraction (e.g. synaptic failure, see above) but slightly increased during the contraction (potentiation effect), independently of the environmental conditions. Even if spinal modulation cannot be ruled out, this suggests that the additional decrease in VA when contraction was prolonged in hyperthermia (Fig. 5) was not due to additional peripheral perturbations but rather to a failure of the CNS at the supraspinal level. This supports a recent study that utilized motor cortex stimulation to show that hyperthermia leads to a failure at or above the level of motor cortical output when contraction is prolonged (Todd et al. 2005). These authors failed to observe this alteration during brief MVC or at the beginning of the 120 s MVC; however, the failure becomes evident after 25 s of contraction in passive hyperthermia (Todd et al. 2005).
Conclusion
In conclusion, our data confirmed that passive hyperthermia reduced voluntary force production partly due to a deficit in muscle activation. This study showed, for the first time, that this deficit can be linked to peripheral failures in the transmission of the neural drive at both spinal and neuromuscular junction levels (e.g. synaptic failures). When contraction was prolonged, additional failures at the supraspinal level seemed to impair motor drive. The presence of cerebral alterations with hyperthermia was confirmed by a decrease in working memory tests. This decrement suggested the existence of frontal lobe activity impairments and can partly be protected by applying cool packs on the head.
Acknowledgments
We thank Ivana Matic for her help in data collection. We thank Nicola A. Maffiuletti for his comments on the manuscript and Farid El Massioui for his help in protocol design.
References
- Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol. 2002;92:2309–2318. doi: 10.1152/japplphysiol.01185.2001. [DOI] [PubMed] [Google Scholar]
- Aird JW, Webb RD, Hoare J. Heat exposure-induced changes in motor outflow component of reaction time. Percept Mot Skills. 1983;56:699–706. doi: 10.2466/pms.1983.56.3.699. [DOI] [PubMed] [Google Scholar]
- Amos D, Hansen R, Lau WM, Michalski JT. Physiological and cognitive performance of soldiers conducting routine patrol and reconnaissance operations in the tropics. Mil Med. 2000;165:961–966. [PubMed] [Google Scholar]
- Andersen B, Westlund B, Krarup C. Failure of activation of spinal motoneurones after muscle fatigue in healthy subjects studied by transcranial magnetic stimulation. J Physiol. 2003;551:345–356. doi: 10.1113/jphysiol.2003.043562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avela J, Finni J, Komi PV. Excitability of the soleus reflex arc during intensive stretch-shortening cycle exercise in two power-trained athlete groups. Eur J Appl Physiol. 2006;97:486–493. doi: 10.1007/s00421-006-0209-6. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OC. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol. 1986;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilodeau M. Central fatigue in continuous and intermittent contractions of triceps brachii. Muscle Nerve. 2006;34:205–213. doi: 10.1002/mus.20572. [DOI] [PubMed] [Google Scholar]
- Caputa M, Feistkorn G, Jessen C. Effects of brain and trunk temperature on exercise performance in goats. Pflugers Arch. 1986;406:184–189. doi: 10.1007/BF00586681. [DOI] [PubMed] [Google Scholar]
- Cian C, Barraud PA, Melin B, Raphel C. Effects of fluid ingestion on cognitive function after heat stress or exercise induced dehydration. Int J Psychophysiol. 2001;42:243–251. doi: 10.1016/s0167-8760(01)00142-8. [DOI] [PubMed] [Google Scholar]
- Desruelle AV, Candas V. Thermoregulatory effects of three different types of head cooling in humans during a mild hyperthermia. Eur J Appl Physiol. 2000;81:33–39. doi: 10.1007/PL00013794. [DOI] [PubMed] [Google Scholar]
- Dewhurst S, Riches PE, Nimmo MA, De Vito G. Temperature dependence of soleus H-reflex and M-wave in young and older women. Eur J Appl Physiol. 2005;94:491–499. doi: 10.1007/s00421-005-1384-6. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Balestra C, Carpentier A, Hainaut K. Reflex regulation during sustained and intermittent submaximal contractions in humans. J Physiol. 2002;541:959–967. doi: 10.1113/jphysiol.2002.016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Behaviour of short and long latency reflexes in fatigued human muscles. J Physiol. 1993;471:787–799. doi: 10.1113/jphysiol.1993.sp019928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A, Carter RA, Mitchell D. Brain and abdominal temperatures at fatigue in rats exercising in the heat. J Appl Physiol. 1998;84:877–883. doi: 10.1152/jappl.1998.84.3.877. [DOI] [PubMed] [Google Scholar]
- Galloway PH, Sahgal A, McKeith IG, Lloyd S, Cook JH, Ferrier IN, Edwardson JA. Visual pattern recognition memory and learning deficits in senile dementias of the Alzheimer and Lewy body types. Dementia. 1992;3:101–107. doi: 10.1001/archneur.1992.00530340059019. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol. 1996;490:529–536. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland SJ. Role of small diameter afferents in reflex inhibition during human muscle fatigue. J Physiol. 1991;435:547–558. doi: 10.1113/jphysiol.1991.sp018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland SJ, McComas AJ. Reflex inhibition of human soleus muscle during fatigue. J Physiol. 1990;429:17–27. doi: 10.1113/jphysiol.1990.sp018241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking C, Silberstain RB, Lau WM, Stough C, Roberts W. Evaluation of cognitive performance in the heat by functional brain imaging and psychometric testing. Comp Biochem Physiol. 2001;128:719–734. doi: 10.1016/s1095-6433(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Joyce E, Blumenthal S, Wessely S. Memory, attention, and executive function in chronic fatigue syndrome. J Neurol Neurosurg Psychiatry. 1996;60:495–503. doi: 10.1136/jnnp.60.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanithi S, Barclay JW, Robertson RM, Brown IR, Atwood HL. Neuroprotection at Drosophila synapses conferred by prior heat shock. J Neurosci. 1999;19:4360–4369. doi: 10.1523/JNEUROSCI.19-11-04360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelty JD, Noseworthy PA, Feder ME, Robertson RM, Ramirez JM. Thermal preconditioning and heat-shock protein 72 preserve synaptic transmission during thermal stress. J Neurosci. 2002;22:RC193. doi: 10.1523/JNEUROSCI.22-01-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris T, Swain J, Smith M, Corbett J, Delves S, Sale C, Harris RC, Potter J. Heat stress, plasma concentrations of adrenaline, noradrenaline, 5-hydroxytryptamine and cortisol, mood state and cognitive performance. Int J Psychophysiol. 2006;61:204–215. doi: 10.1016/j.ijpsycho.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Malhotra P, Jäger HR, Parton A, Greenwood R, Playford ED, Brown MM, Driver J, Husain M. Spatial working memory capacity in unilateral neglect. Brain. 2005;128:424–435. doi: 10.1093/brain/awh372. [DOI] [PubMed] [Google Scholar]
- Mariak Z, White MD, Lyson T, Lewko J. Tympanic temperature reflects intracranial temperature changes in humans. Pflugers Arch. 2003;446:279–284. doi: 10.1007/s00424-003-1021-3. [DOI] [PubMed] [Google Scholar]
- Marino FE, Lambert MI, Noakes TD. Superior performance of African runners in warm humid but not in cool environmental conditions. J Appl Physiol. 2004;96:124–130. doi: 10.1152/japplphysiol.00582.2003. [DOI] [PubMed] [Google Scholar]
- Martin PG, Marino FE, Rattey J, Kay D, Cannon J. Reduced voluntary activation of human skeletal muscle during shortening and lengthening contractions in whole body hyperthermia. Exp Physiol. 2005;90:225–236. doi: 10.1113/expphysiol.2004.028977. [DOI] [PubMed] [Google Scholar]
- Morris RG, Downes JJ, Sahakian BJ, Evenden JL, Heald A, Robbins TW. Planning and spatial working memory in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:757–766. doi: 10.1136/jnnp.51.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S, Sleivert GG, Cheung SS. Passive hyperthermia reduces voluntary activation and isometric force production. Eur J Appl Physiol. 2004;91:729–736. doi: 10.1007/s00421-004-1063-z. [DOI] [PubMed] [Google Scholar]
- Mündel T, Hooper PL, Bunn SJ, Jones DA. The effects of face cooling on the prolactin response and subjective comfort during moderate passive heating in humans. Exp Physiol. 2006;91:1007–1014. doi: 10.1113/expphysiol.2006.034629. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Hyldig T, Bidstrup F, González-Alonso J, Christoffersen GR. Brain activity and fatigue during prolonged exercise in the heat. Pflugers Arch. 2001;442:41–48. doi: 10.1007/s004240100515. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B. Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol. 2001;91:1055–1060. doi: 10.1152/jappl.2001.91.3.1055. [DOI] [PubMed] [Google Scholar]
- Nybo L, Secher NH, Nielsen B. Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J Physiol. 2002;545:697–704. doi: 10.1113/jphysiol.2002.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1995;33:1–24. doi: 10.1016/0028-3932(94)00098-a. [DOI] [PubMed] [Google Scholar]
- Racinais S, Bishop D, Denis R, Lattier G, Mendez-Villaneuva A, Perrey S. Muscle de-oxygenation and neural drive to the muscle during repeated sprint cycling. Med Sci Sports Exerc. 2007a;39:268–274. doi: 10.1249/01.mss.0000251775.46460.cb. [DOI] [PubMed] [Google Scholar]
- Racinais S, Girard O, Micallef JP, Perrey S. Failed excitability of spinal motoneurons induced by prolonged running exercise. J Neurophysiol. 2007b;97:596–603. doi: 10.1152/jn.00903.2006. [DOI] [PubMed] [Google Scholar]
- Ramsey JD, Burford CL, Beshir MY, Jensen RC. Effects of workplace thermal conditions on safe work behavior. J Safety Res. 1983;14:105–114. [Google Scholar]
- Rutkove SB. Effects of temperature on neuromuscular electrophysiology. Muscle Nerve. 2001;24:867–882. doi: 10.1002/mus.1084. [DOI] [PubMed] [Google Scholar]
- Simmons SE, Mündel T, Jones DA. The effects of passive heating and head-cooling on perception of exercise in the heat. Eur J Appl Physiol. 2008;104:282–288. doi: 10.1007/s00421-007-0652-z. [DOI] [PubMed] [Google Scholar]
- Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn BD, Iddon JL, Robbins TW, Sahakian BJ. Early detection and differential diagnosis of Alzheimer's disease and depression with neuropsychological. Dement Geriatr Cogn Disord. 2001;12:265–280. doi: 10.1159/000051269. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Petersen N, Butler JE, Gandevia SC. Ischaemia after exercise does not reduce responses of human motoneurones to cortical or corticospinal tract stimulation. J Physiol. 2000;525:793–801. doi: 10.1111/j.1469-7793.2000.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Todd G, Gandevia SC. Evidence for a supraspinal contribution to human muscle fatigue. Clin Exp Pharmacol Physiol. 2006;33:400–405. doi: 10.1111/j.1440-1681.2006.04363.x. [DOI] [PubMed] [Google Scholar]
- Thomas MM, Cheung SS, Elder GC, Sleivert GC. Voluntary muscle activation is impaired by core temperature rather than local muscle temperature. J Appl Physiol. 2006;100:1361–1369. doi: 10.1152/japplphysiol.00945.2005. [DOI] [PubMed] [Google Scholar]
- Todd G, Butler JE, Taylor JL, Gandevia SC. Hyperthermia: a failure of the motor cortex and the muscle. J Physiol. 2005;563:621–631. doi: 10.1113/jphysiol.2004.077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R, Marle T, Lambert EV, Noakes TD. The rate of heat storage mediates an anticipatory reduction in exercise intensity during cycling at a fixed rating of perceived exertion. J Physiol. 2006;574:905–915. doi: 10.1113/jphysiol.2005.101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R, Rauch L, Harley YXR, Noakes TD. Impaired exercise performance in the heat is associated with an anticipatory reduction in skeletal muscle recruitment. Pflugers Arch. 2004;448:422–430. doi: 10.1007/s00424-004-1267-4. [DOI] [PubMed] [Google Scholar]
- Upton ARM, McComas AJ, Sica REP. Potentiation of ‘late’ responses evoked in muscles during effort. J Neurol Neurosurg Psychiatry. 1971;34:699–711. doi: 10.1136/jnnp.34.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JJ, Furbush F, Bigland-Ritchie B. Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. J Neurophysiol. 1987;58:125–137. doi: 10.1152/jn.1987.58.1.125. [DOI] [PubMed] [Google Scholar]
- Zehr EP. Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol. 2002;86:455–468. doi: 10.1007/s00421-002-0577-5. [DOI] [PubMed] [Google Scholar]