Abstract
Recently, several studies demonstrated the feasibility of a real-time quantitative PCR (qPCR) approach for chimerism monitoring. qPCR offers a fast, sensitive, and elegant quantification of genotypes. However, before it becomes an established method for routine chimerism monitoring, a qPCR marker set for every transplant pair should be available. This requirement poses a major challenge since the genetic markers for qPCR— short insertions/deletions (Indels) and single nucleotide polymorphisms (SNPs)—published to-date do not guarantee applicability for every transplant pair. The aim of our study was to design and validate a new SNP allele-specific system to supplement an already existing Indel primer panel and improve applicability of the qPCR approach for chimerism status monitoring. Here, we present an approach for an economical in-house design of SNP allele-specific qPCR primers/probe sets with a locus-individualized reference system that allows for the accurate quantification of the respective informative locus using a simple ΔΔCt method. We designed primers/probe sets specific for seven biallelic SNP loci and validated them in a population of 30 transplant pairs. Repeatability varied depending on the amount of quantifiable genotype. The combination of our SNP-qPCR system and Indel primers increased recipient genotype identification from 86.6% to 96.6% when tested in a population of our transplant pairs. These results demonstrate the feasibility of our SNP-based qPCR approach to improve the applicability of a qPCR for chimerism monitoring.
Bone marrow transplantation (BMT) is an established medical procedure used to treat various malignant and nonmalignant hematological diseases such as leukemia, aplastic anemia, and lymphoma. In patients suffering from hematopoietic disorders, bone marrow may be affected by malignancy or the malfunction of hematopietic stem cells. The success of a BMT procedure will rely on engraftment of the donor's transplant and restoration of deficient hematopoiesis. The major causes of treatment failure are disease relapse, graft rejection, and graft-versus-host disease. The key test used to predict disease relapse and graft rejection is monitoring of post-transplant chimerism. This assay allows the proportion of recipient and donor cells to be determined, and thus it can be ascertained whether donor engraftment has occurred and whether there are residual recipient cells that may be responsible for a relapse.1,2
Various chimerism assessment techniques have been described so far.3,4 The basic principle in the detection of chimerism is the utilization of differences between recipient and donor genomes. PCR based methods based on genome identity of individuals or unique genetic markers that characterize each individual are preferred because PCR amplification is a rapid, robust, and sensitive technique. A number of types of genetic markers can be used for chimerism evaluation, including variable number of tandem repeats and short tandem repeats.3,4 The short tandem repeat genotyping method is the most widely used for chimerism assessment because of its high informativity, but short tandem repeats are repetitive sequences not suitable for allele-specific real-time quantitative PCR. An alternative approach employs a true quantitative PCR principle (qPCR) and different types of polymorphic genetic markers, single nucleotide polymorphisms (SNP)5 and short insertion/deletion (Indel).6 Single nucleotide polymorphism is the most abundant form of genetic variability in the human genome. A SNP is a single base substitution of one nucleotide by another, and both versions are observed in the general population at a frequency greater than 1%. Any two individuals are predicted to vary by more than a million different SNPs dispersed through the genome.7 SNPs have proved to be particularly useful as markers for monitoring chimerism after bone marrow transplantation because they are stable and unique and can be analyzed by sensitive quantitative methods.5
In our unit, the Indel polymorphism-based qPCR system has been used for genotyping recipient/donor pairs and for chimerism assessment.6 Indel sets are useful to distinguish the vast majority of recipient genotypes; however, clinical practice requires an identification marker for every patient to be monitored by qPCR. To search for informative markers, donor/patient pairs were additionally screened with SNP-based genotyping system from Maas and coauthors.8 Unfortunately, this system showed low applicability in our population of transplant pairs where Indel sets failed to reveal an informative marker of the recipient. Therefore, alternative markers are needed to complement Indel sets to allow qPCR chimerism analysis for our patients.
In the current study, we describe a new panel of seven SNP loci and an innovative approach for the locus-specific endogenous control to be used for chimerism monitoring by qPCR.
Materials and Methods
Patients, Donors, and Samples
Samples from 30 patients undergoing BMT for chronic myeloid leukemia (n = 8), acute myeloid leukemia (n = 8), lymphoma (n = 4), acute lymphoblastic leukemia (n = 4), myelofibrosis (n = 2), aplastic anemia (n = 3), and lymphohistiocytic hemaphagocytosis (n = 1) were analyzed. The donors for these patients were either human leukocyte antigen-matched siblings (n = 14) or human leukocyte antigen-matched unrelated individuals (n = 16).
Donor samples and pre-BMT recipient samples were used for screening of genetic markers for chimerism analysis. Post-BMT samples were retrospectively analyzed to assess the usefulness of the qPCR chimerism assay.
Genomic DNA was extracted from blood or bone marrow using a QIAmp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to manufacturer's instructions. The concentration and purity of each DNA sample was evaluated spectrophotometrically.
The study was approved by the Lithuanian Bioethics Committee.
SNPs Selection and Primer and Probe Design
SNP loci were selected from the dbSNP database (http://www.ncbi.nlm.nih.gov/SNP). First, biallelic polymorphisms with allele frequency of 0.4 to 0.6 and A/G or C/T alleles were used. Additionally, SNP loci were selected by the virtual availability to design suitable primer sets for a qPCR assay. An allele-specific primer, common opposite primer, and common dual-labeled probe format was selected for generation of SNP-detection primer/probe sets. PCR primers were designed according to amplification refractory mutation system (ARMS) technology, which was originally developed by Newton and coauthors9,10 using Primer3 design (v. 0.4.0) software (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi), Operon biotechnologies oligo calculation tool (https://www.operon.com/oligos/toolkit.php) and RealTimeDesign software (http://www.biosearchtech.com/products/probe_design.asp). The thermodynamic properties of amplification regions were checked with the DINAMelt software (http://www.bioinfo.rpi.edu/applications/hybrid/twostate-fold.php).
Initially, for each SNP allele, two different allele-specific ARMS primers were designed with one or two mismatches at the 3′ end of the oligonucleotide. The discriminatory power of both types of allele-specific primers was first determined by qualitative PCR under the same conditions as those required for the qPCR. This analysis was done using the DNA samples of 30 transplant pairs. The allele discriminating primer sets were determined by unambiguous presence/absence of PCR products between individuals.
Allele-specific primers were tested by the dual-labeled hydrolysis probe qPCR. A specific threshold cycle (Ct) was determined for each primer/probe set. Positive alleles generated Ct values ranging from 25 to 36. Finally, a panel of polymorphic markers was assembled of seven biallelic SNPs. The sequences of primer and probe sets are listed in Table 1.
Table 1.
Characteristics of SNP Marker Panel
| dbSNP ID location | Nucleotide variation | 5′-Primer-3′ | Hydrolysis probe (6FAM-BHQ) | Individual informativity of SNP alleles | PCR product length (bp) |
|---|---|---|---|---|---|
| rs715463 | C | F_5′-GGGGCTTATGCCTCAAGACAC-3′ | R_5′-CAACTTATTCCTGGTC | 20% | 103 |
| 14q24.2 | T | F_5′-GGGGCTTATGCCTCAAGACAT-3′ | AGTGTCCCGCAT-3′ | 13% | |
| R*_5′-ACCCAGCTTTCCTTCATTGG-3′ | |||||
| Uni_F_5′-GAAAAGGGGCTTATGCCTCAAAATA-3′ | 104 (uni) | ||||
| rs715405 | G | R_5′-CAGGCATTATATTTAGGCTAAC-3′ | F_5′-CTGACATTCAAAGACT | 4% | 84 |
| 1p31 | A | R_5′-CAGGCATTATATTTAGGCTAAT-3′ | GCTCACTGACAAAG-3′ | 16% | |
| F*_5′-TCCACAGGG TTATCAAGTTGTTC-3′ | |||||
| Uni_R_5′-AATCAGGCATTATATTTAGGCTGA-3′ | 87 (uni) | ||||
| rs714825 | C | R_5′-CTTCCTGATTAGTTAGTTGAATGAATG-3′ | R_5′-TCTCAGTCCTCATGT | 23% | 90 |
| 4q28 | T | R_5′-CTTCCTGATTAGTTAGTTGAATGAATA-3′ | ACAAGGTGCTGTTC-3′ | 10% | |
| F*_5′-CCATTTTTATGTCCTATACTTTCAGTG-3′ | |||||
| Uni_R_5′-GCTTCCTGATTAGTTAGTTGAATAAGT-3′ | 90 (uni) | ||||
| rs714421 | G | F_5′-CTATAAGCAAGAGTCCATTAAG-3′ | R_5′-CCTGCTGTGTATCAA | 20% | 140 |
| 14q24 | A | F_5′-CTATAAGCAAGAGTCCATTAAA-3′ | AGCTGGGTACTCA-3′ | 16% | |
| R*_5′-ATCGTGCAGCCTTCCTGA-3′ | |||||
| Uni_F_5′-CCTATAAGCAAGAGTCCATTAG-3′ | 140 (uni) | ||||
| rs714215 | C | F_5′-CTCACTCTCCATCCTTTC-3′ | R_5′-CTGTCCTGCCACACAT | 0.6% | 86 |
| 11p11 | T | F_5′-CTCACTCTCCATCCTCCT-3′ | GGTCCCA-3′ | 46% | |
| R*_5′-AGCCTGTTCTTCTCTTCCTG-3′ | |||||
| Uni_F_5′-CCTCACTCTCCATCCTTC-3′ | 86 (uni) | ||||
| rs713753 | C | F_5′-TCAAAGGTGGGGAATCGAC-3′ | F_5′-CCTGCCCAAAGGCCCA | 30% | 97 |
| 22q13 | T | F_5′-TCAAAGGTGGGGAATCGAT-3′ | GAGAGAC-3′ | 23% | |
| R*_5′-GCTGACTCTTGCTCCATC-3′ | |||||
| Uni_F_5′-GTCAAAGGTGGGGAACCAA-3′ | 96 (uni) | ||||
| rs713503 | G | F_5′-GACCATGCCTTGCTTTTATG-3′ | R_5′-CACCTACTCCTATTGCTT | 14% | 98 |
| 11p15 | T | F_5′-GACCATGCCTTGCTTTTATT-3′ | GAAGGAAACACG-3′ | 22% | |
| R*_5′-CCTTTTGTTCACGTCCCTCA-3′ | |||||
| Uni_F_5′-GGACCATGCCTTGCTTTTGT-3′ | 97 (uni) |
SNP loci were selected from the dbSNP database (http://www.ncbi.nlm.nih.gov/SNP).
Sequence of probes and primers, nucleotide variations, and percentage of informativity of individual alleles are indicated. Polymorphic nucleotides are given in bold and mismatched nucleotides introduced are bold underlined. Probes and asterisk-labeled (*) primers are common for both alleles.
F_ indicates forward primer/probe; R_ stands for reverse primer/probe; Uni_ denotes locus universal primers.
Reference System
One of the most important factors in relative quantitation by qPCR is the selection of an appropriate endogenous control, which is often referred to as the housekeeping gene. For the quantification of target genotype we implemented an alternative locus-individualized endogenous control with genomic position overlapping with the sequence of the respective informative SNP. Thus, for each SNP locus an additional universal primer was synthesized that was shifted upstream from the dimorphic site by one to several nucleotides leaving the same opposite common primer and a common probe used for informative allele amplification. Therefore, the amount of endogenous control quantified in this manner reflects the total amount of a certain SNP locus while for the amplification with the allele-specific primer, the same opposite common primer and a probe measures the amount of informative allele within that locus. The sequences of SNP loci-universal primers are specified in Table 1.
Quantitative Real-Time PCR Assay
qPCR was performed on a RotorGene 6500-HRM Real-time PCR instrument (Corbett Research, Mortlake, Australia). PCR conditions were as follows: 100 ng genomic DNA, 12.5 μl QuantiTect Probe PCR Master Mix 2× (Qiagen, Hilden, Germany), and 300 nmol/L of each primer and 200 nmol/L of probe (Operon Biotechnologies, Cologne, Germany) in a final volume of 25 μl. Screening and quantification assays were performed with standard cycling conditions: 2 minutes at 50°C followed by 5 minutes at 95°C and 50 amplification cycles at 95°C for 15 seconds, and 60°C for 60 seconds.
Standard Amplification Curves
To evaluate the detection limit of the qPCR assay, serial dilutions of each allele-positive DNA template in allele-negative DNA (1:1 to 1:10.000) were amplified. The input of allele-positive DNA and the total amount of chimeric DNA mixture within each dilution was 100 ng. All PCR reactions were performed in triplicate. Standard amplification curves were plotted for each pair of ARMS-SNP detection primers using Rotor-Gene 6000 Series Software version 1.7.34. The generation of typical amplification curves was used to determine the detection limit for each ARMS-SNP primer pair.
Serial dilution experiments were also performed for the evaluation of amplification efficiencies of informative allele and its respective endogenous control. Each informative allele-positive DNA template was tenfold serially diluted (1:1 to 1:10.000) and tested by qPCR using SNP allele-specific and respective endogenous control primer/probe sets. Amplification curves were plotted using Rotor-Gene 6000 Series Software version 1.7.34. Amplification efficiencies of both informative allele and respective endogenous control were automatically calculated by software.
qPCR Chimerism Assay
Marker Screening
To evaluate the informativity of SNP markers, recipients and their donors were genotyped using ARMS-SNP detection primers. SNP markers were considered informative for recipient genotype if positive on recipient DNA and negative on donor DNA and were considered informative for donor genotype if positive on donor DNA and negative on recipient DNA. Thus the first informative allele selection criterion was presence of allele within recipient/donor genome either in homozygous or heterozygous state. Zygocity state of informative allele or alleles was taken into account for chimerism result quantification.
A specific Ct was determined for each PCR. Only primer sets with Ct values up to 30 cycles were used for chimerism monitoring to reach at least 0.1% detection limit of the assay.
Informativity of individual SNP alleles was calculated as a percentage of pairs where discrimination between recipient and donor was possible in our model population of 30 transplant pairs.
Chimerism Evaluation
An SNP qPCR chimerism assay was performed retrospectively on pre-BMT recipient and donor DNAs and post-BMT DNA samples. One recipient-specific allele and the respective endogenous control were used for chimerism quantification in post-BMT samples. A real-time quantitative PCR chimerism assay was performed in duplicate and under the same PCR conditions as outlined above. The relative proportion of recipient-unique allele in the post-BMT sample was calculated using the ΔΔCt method of relative quantification as described.6
To assess the variability of quantification results using different recipient-unique alleles, the retrospective SNP qPCR chimerism analysis was performed on post-BMT patient samples. Statistical analysis was performed using GraphPad Prism Version 5.0 (GraphPad Software Inc., San Diego).
Quantification Method
The amplification efficiency of each SNP marker and its respective endogenous control was tested by means of standard curve amplification using Rotor-Gene 6000 Series Software version 1.7.34.
The amount of informative allele was assessed by relating the Ct value of informative allele to the Ct value of its respective endogenous control both in pre-BMT sample (calibrator ΔCtC) and in post-BMT sample (unknown ΔCtU) The quantity of informative allele was calculated as follows:
where ΔCtU is ΔCt in the unknown sample ΔCtC is ΔCt in the calibrator sample; E is PCR efficiency.
If a heterozygous marker was used for chimerism quantification, the result was multiplied by 2.
Comparison of Real-time PCR Chimerism Quantification Systems
To evaluate the relationship between SNP qPCR and Indel qPCR6 chimerism quantification systems, a correlation analysis for each set of post-BMT results was performed. The values of each quantification system were log-transformed and a scatter plot of a linear regression model was generated. Agreement between the two measurement techniques was assessed using Bland and Altman statistics. Statistical analysis was performed using GraphPad Prism Version 5.0 (GraphPad Software Inc., San Diego).
Results
In-House Design of a SNP-Based Genotyping System for Chimerism Status Evaluation after BMT
For quantification of mixed chimerism by dual-labeled hydrolysis probe qPCR, SNPs were selected from the dbSNP database (http://www.ncbi.nlm.nih.gov/SNP). Aiming to assemble the most informative SNPs, we selected only biallelic loci with allele frequency of 0.4 to 0.6. SNP loci with C/T and G/A nucleotide variations were selected for better allele discrimination. An additional locus selection criterion was suitability to design primer and probe sets, compatible with dual-labeled hydrolysis probe qPCR. The ARMS PCR approach was applied for SNP-detection primer design.8,11 Original ARMS technology uses allele-specific primers containing one additional mismatched nucleotide located at the 3′ end of the primer three nucleotides from polymorphic nucleotide. Our allele-specific primers contain one or two additional noncomplementary nucleotides located one to four positions upstream from dimorphic site (Table 1). Mismatched nucleotide, ie, A, C, G, or T, and position at which nucleotide was introduced were determined by the most optimal primer properties provided by on-line primer design tools. Screening of 30 transplant pairs with our SNP allele-specific primer/probe sets revealed positive alleles at Ct within 25 to 36 cycles with no background amplification.
For reliable quantification of target genotype, we developed an alternative reference system individualized for each SNP locus. The amplification efficiency of each informative and its respective endogenous control was evaluated by means of standard curve amplification and found to be highly comparable (ΔE ≤0.1). Moreover, the principle of alternative reference system was also adapted for Indel markers.6 The developed universal primer sequences for Indel marker panel are provided in Table 2.
Table 2.
Locus-Universal Primers for Indel Marker Panel
| Marker name | 5′-Primer-3′ |
|---|---|
| S01_Uni_F | 5′-GTGAGGCTGCTGGGTCGTG-3′ |
| S02_Uni_F* | 5′-CTGCTGCTTCTCTGGTTGGAG-3′ |
| S02_Uni_R* | 5′-TCAGTGCTTGCTGGCGGAC-3′ |
| S03_Uni_R | 5′-AAATCAATCTTTGGGCAGGTTG-3′ |
| S04_Uni_R | 5′-CCATAAGGATGCGTGACTGCT-3′ |
| S05_Uni_F | 5′-CTGGGCAGCCATTTTTCTTCTCTG-3′ |
| S06_Uni_F | 5′-CAGCCACCAGTCACCCTATG-3′ |
| S07_Uni_F | 5′-GAAGTCTGGTATTGGCTTTAAAATAC-3′ |
| S08_Uni_F | 5′-CTAAGCTGGATGCCTCACTGA-3′ |
| S09_Uni_R | 5′-GGCTCAGCTTGTCTGCTTTCT-3′ |
| S10_Uni_F | 5′-GCCATTTTAGAGCCACAAGAGAC-3′ |
| S11_Uni_F | 5′-CATGGAGCTAGGATTCAACC-3′ |
Universal primers for S02 should be used for chimerism analysis if both the recipient and the donor are of male gender.
Informativity of the SNP Panel
To determine the capacity of the SNPs to discriminate between individuals, 30 recipient and donor pairs were screened for the presence of the fourteen SNPs. Informative allele or alleles were found in each recipient/donor pair tested, irrespective of relatedness or sex. Namely, recipient genotype discrimination was possible in 20/30 (66.7%) pairs tested (7/14 [50%] related pairs and 13/16 (81.5%) unrelated pairs), while donor genotype was distinguished in 26/30 (86.7%) pairs (13/14 [92.8%] related pairs, and 13/16 [81.5%] unrelated pairs). We identified 1 to 6 informative alleles for each recipient/donor pair. One informative SNP marker was found in 3 transplant pairs, 2 in 16 pairs, 3 in 5 pairs, 4 in 3 pairs, 5 in 1 pair, and 6 informative markers were found in 2 transplant pairs. Of these, for recipient genotype 7 cases had 1 informative SNP, 9 cases, 2 SNPs, and 3 cases, 3 SNPs. For donor genotype 12 cases had 1 SNP, 11 cases, 2 SNPs, and 2 cases, 4 SNPs. Informativity of SNP alleles calculated as a percentage of pairs where discrimination by individual allele was possible ranged from 1% to 46% within the tested population (Table 1).
The discrimination of both genotypes was detected in 17 out of 30 recipient/donor pairs. The overall informativity of our set of seven SNP loci was 57% (37% in unrelated pairs and 20% in siblings).
Detection Limit of the Assay
To determine the detection limit of our qPCR assay, each allele-specific primer set was tested for sensitivity using a dilution series of the informative template in allele-negative template. Each qPCR reaction was performed in triplicate. The threshold line was set automatically by instrument software. Figure 1 shows a typical amplification plot of one SNP marker obtained from a dilution experiment. Mean reproducibility of Ct values of each triplicate was 0.06 (ranged from 0.02 to 0.3). As expected, each log dilution of target DNA was detected by an additional three cycles of qPCR (range 2.64 to 3.25). Our SNP qPCR genotyping system was able to detect a positive allele within 10 cycle interval (ΔCt ≤10) of allele-specific qPCR and thus allowed quantification of the target template down to 0.1%. No amplification was detected for 0.01% target DNA and allele-negative templates up to cycle 50 (Supplemental Figure S1 available at http://jmd.amjpathol.org).
Figure 1.
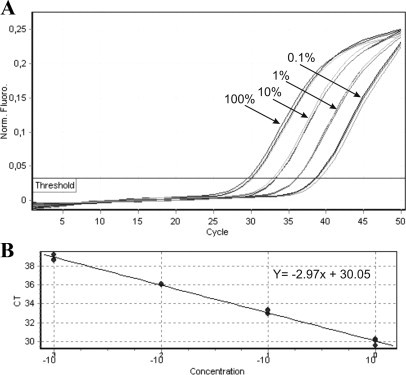
Evaluation of SNP RQ-PCR sensitivity and accuracy. A: Fluorescence versus cycle plots were constructed from serial dilutions of 100, 10, 1, 0.1, and 0.01% of positive patient DNA in negative donor DNA. The total amount of DNA mix was 100 ng. The lowest concentration of SNP-positive template reliably detected in all samples was 0.1%. B: A standard amplification curve was generated by plotting the cycle number versus the concentration. The correlation coefficient between the calculated and the known concentrations was 0.99. Amplification efficiency calculated from the slope of the curve was 1.17.
Evaluation of SNP qPCR Chimerism Quantification System
To confirm that our SNP panel reliably detects chimerism status, we performed retrospective analysis on samples from six patients whose chimerism status had been monitored previously using the Indel qPCR system6 for at least six months. The recipient genotype proportion in these samples ranged from 0% to 100%.
Chimerism status in the post-transplant samples of each patient was reanalyzed and quantified using our SNP qPCR system. For quantification, one informative recipient marker and the respective endogenous control were used. Recipient genotype was quantified as outlined in the Materials and Methods.
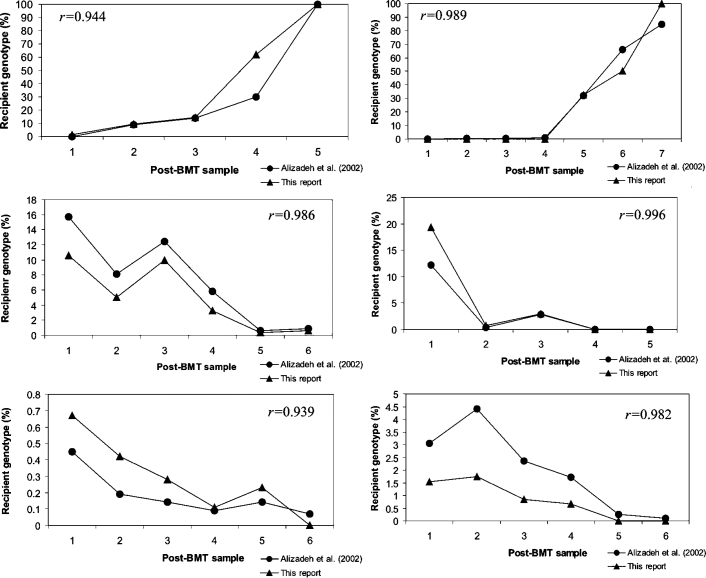
Comparison of both chimerism assessment systems revealed that our qPCR method achieved concordant results in all post-BMT samples tested. Correlation coefficients (r) calculated for each individual set of chimerism results ranged from 0.939 to 0.996 (Figure 2). Furthermore, a linear regression model generated from these data showed that the two Real-time PCR assay systems correlated well (r = 97.44%; 95% CI ± 0.05) (Figure 3A). The overall SD was 26.06%. To evaluate the repeatability of chimerism values obtained by both qPCR chimerism quantification systems, we stratified the patients into two groups: one with the decreasing (from 15% to 0%) and the other with the increasing recipient genotype (from 0.06% to 100%). Statistical analysis of the chimerism results obtained in samples with decreasing recipient profile showed excellent correlation (r = 98.3%; 95% CI ± 0.05) and repeatability with mean SD 3.43%. The variability of chimerism values obtained in samples with increasing recipient profile was significantly higher with mean SD 35.2% (r = 95.6%, 95% CI ± 0.9).
Figure 2.
Comparison of chimerism quantification results obtained with short insertion/deletion polymorphism qPCR6 and SNP-specific qPCR (this report) for six different BMT patients. Six different SNP alleles were used for chimerism evaluation by SNP-specific qPCR: rs714215 variant T, rs714421 variant A, rs713503 variant G, rs715405 variant G, rs715405 variant A, and rs713753 variant C.
Figure 3.
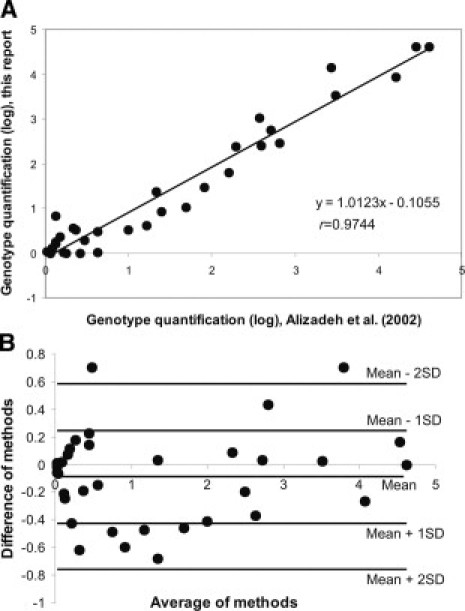
Scatterplots with regression analysis (A) and Bland Altman analysis (B) of SNP-specific qPCR versus short insertion/deletion polymorphism qPCR6 (n = 35).
Bland Altman Analysis
To measure the strength of relation between the two chimerism assessment systems, Bland and Altman statistics were applied.12,13 The clinically acceptable limits of variability between techniques were calculated as mean ± 2SD. The resulting scatter diagram of the first and second techniques (Figure 3B) showed limits of agreement between −0.569 and 0.745 on the log scale. Calculated antilogs of these limits were 0.566 and 2.10, respectively (ie, in 95% of cases the SNP RQ-PCR results were between 0.566 and 2.10 times those of the Indel qPCR results). The calculated bias was 0.088.
Correlation of Quantification of Chimerism Using Different SNP Alleles
We selected seven patients whose successive chimerism values varied over the monitoring period. In total, 48 post-transplant samples were available from these patients, namely 7 samples from patient 1 with rs714421-A and rs714215-T; 8 samples from patient 2 with rs714421-A and rs713503-T; 7 samples from patient 3 with rs714215-T, rs713753-C, and rs713503-T; 6 samples from patient 4 with rs715405-G, rs714825-C, and rs713503-G; 8 samples from patient 5 with rs715463-C and rs714421-G; 8 samples from patient 6 with rs714215-T and rs713753-C, and 6 samples from patient 7 with rs715463-T and rs713753-T.
Recipient genotype was quantified as described above.
The quantity of the recipient genotype calculated using different recipient-informative alleles showed good correlation with coefficient ranging form 94.5% to 99.91% and mean SD 18.5%. Then the patients were assigned into two groups with decreasing (17.1% to 0%) or increasing (0.1% to 100%) recipient genotype. The estimated SD in post-BMT samples with decreasing recipient profile was 3.4% (95% CI ± 0.03, r = 99.8%). As expected, the SNP qPCR assay was significantly less accurate in quantifying target genotype at increasing levels. The SD in this group ranged from 19.4% to 34.6% (95% CI ± 1.2, r = 96.5%).
Discussion
Analysis of chimerism after allogeneic bone marrow transplantation is important for the assessment of engraftment kinetics and the early detection of graft failure. Chimerism assessment is a quantitative determination of proportion of recipient and donor genotypes within the newly developed hematopoietic system based on distinct genetic identities of the recipient and the donor. A number of methods using repetitive sequence polymorphisms are described for chimerism evaluation. Recently different types of genome polymorphisms were suggested for more sensitive chimerism monitoring by qPCR. Originally, Oliver and coauthors5 showed suitability of SNP for qPCR chimerism evaluation. Later, Alizadeh and coauthors6 demonstrated feasibility of Indel markers for this purpose.
Single nucleotide polymorphisms provide an almost unlimited source of human genetic polymorphisms that are unique and can be analyzed by qPCR. Despite the theoretical advantages of SNP application for chimerism assessment, these genetic markers are still of limited use because of the complexity of assay design as well as limited informativity of SNP loci. A biallelic SNP locus has only three possible genotypes, while a single short tandem repeat locus has between 8 and 40 different alleles. Thus, a higher number of SNP loci should be screened to find at least one informative marker for every recipient/donor pair. However, the abundance of SNPs overcomes these limitations, and a panel of SNP allele specific qPCR primers would allow the identification of informative alleles. Virtually any biallelic SNP locus with allele frequencies of 0.4 to 0.6 could be used for genotype identification in a transplant pair, provided primers and probes suitable for qPCR are designed for this locus. On the basis of our experience we suggest that loci with C/T and G/A are the most promising because these nucleotide variations allow more specific allele discrimination by ARMS technology.
In this study we have developed and validated a supplemental SNP marker panel to improve applicability of qPCR for chimerism monitoring in routine practice. To date, Indel markers6 used for informative allele screening allowed recipient genotype identification in 86.6% (113/127) of transplant pairs tested in our unit. Recipient discrimination was increased to 96.6% (123/127) with the new SNP marker panel. This compares favorably with the highest level of informativity reported to date.14
In this study we have adapted the approach of the individualized reference system used for chimerism quantification by ΔΔCt. This method provides reliable quantitative data if the amplification efficiencies of both target and reference genes are similar. PCR efficiency is affected by interrun variation, reagents and instrumentation used. Amplification efficiency is also influenced by DNA sequence of a fragment to be amplified. To achieve most comparable amplification efficiencies of a target and a reference both PCR products should be amplified from the same template and be of the similar length.
We applied this principle in our study since amplification efficiencies of different alleles in our panel varied. For each SNP locus, we designed a universal primer to amplify the target locus while an allele-specific primer is used for amplification of the informative allele. This approach ensures reliable relative quantification and eliminates the necessity to use standard curve amplification within each run. In addition, the developed reference system allows accurate quantification of the respective informative locus. Moreover, the developed SNP-based chimerism assessment system is an economical in-house qPCR system that employs the same dual-labeled probe and opposite common primer for both target and respective endogenous control amplification.
We also designed and implemented locus universal primers for the Indel panel,6 since amplification efficiency of GAPDH reference was significantly lower than that of Indel markers.
An important feature of chimerism assays is their ability to detect low levels of mixed chimerism. Different PCR-based chimerism methods have different detection levels. The detection limit of the semiquantitative microsatellite amplification method is estimated to be 1 to 5%,15,16,17,18 that of pyrosequencing is about 5%,19 and that of the microarray-based method is about 1%.20 To date, qPCR offers the highest sensitivity. Detection of 0.1% of target DNA has been reported for SNP8,14,21 compared with 0.1% to 0.01% for bialellic short insertion/deletion polymorphisms.6,14,22
The detection limit of our SNP-specific qPCR assay was evaluated with dilution experiments of allele-positive DNA in allele-negative DNA. A linear correlation of target DNA template fraction (r = 99%) for all SNP markers was detected down to 0.1% of the test template (Figure 1). Our SNP-specific qPCR assay accurately measured the presence of 0.1% allele-positive template despite the fact that amplification with allele-specific ARMS primers resulted in later Ct compared with amplification with perfectly matched primers (Ct ≤25). This phenomenon is associated with the less efficient ability of Taq polymerase to extend a primer with a mismatch within the 3′-end.23,24 In our assays, the specific threshold cycle for informative allele PCR resulted in Ct ≤30. Thus, the sensitivity of the assay was somewhat restricted, since all amplification curves were shifted to the right by approximately 2 to 7 cycles. Nevertheless, the sensitivity of our SNP qPCR assay is consistent to that reported by others.8,21
Evaluation of our qPCR SNP assay performance for post-transplant monitoring of mixed chimerism showed a very high concordance to that of Indel qPCR (Figures 2 and 3A). Moreover, the developed SNP-based chimerism assessment system comprised of an informative marker and the respective endogenous control primer and probe sets accurately reproduced chimerism values obtained by different informative SNP alleles. The assay repeatability indicates that a single recipient informative marker is fully sufficient for reliable chimerism monitoring. However, due to the nature of the PCR methodology itself, this method is extremely accurate in quantification of a minor marker, whereas it is significantly less so in quantification of a major marker. To overcome this drawback and ensure optimal and accurate chimerism evaluation, this method requires at least one locus that is informative for the recipient cells and one locus that is informative for the donor cells. In the case of nearly complete donor chimerism, the recipient unique marker is used for quantification of residual recipient genotype, while the donor-unique marker is useful for quantification of residual donor cells when chimerism status converts to recipient dominant genotype.
To determine the agreement between Indel-based and SNP-based chimerism assessment methods, Bland-Altman statistics were applied. Analysis has shown 94.28% (ie, 33 out of 35) of data points fall within the two standard deviations of the mean and a bias of 0.088 (Figure 3B). The results indicate that the SNP-based qPCR method agrees well with the existing Indel qPCR method, and that the simple and robust procedure can be included for accurate chimerism assessment in post-BMT samples.
In conclusion, the developed SNP marker panel may contribute to the successful screening for informative markers and further extend the overall number of SNPs available for chimerism evaluation5,8,11,19,20,21 and thus the overall applicability of SNP qPCR in routine chimerism monitoring. Moreover, the locus-individualized endogenous reference allows accurate relative allele quantification without the need of standard curves.
Acknowledgements
We thank Viktor Skorniakov (Information and Technology Center of Vilnius University Hospital Santariskiu Clinics) for advice in performing statistical analysis.
Footnotes
Supplemental material for this article can be found at http://jmd.amjpathol.org.
Supplementary data
References
- 1.Choi SJ, Lee KH, Lee JH, Kim S, Chung HJ, Lee JS, Kim SH, Park CJ, Chi HS, Kim WK. Prognostic value of hematopoietic chimerism in patients with acute leukemia after allogeneic bone marrow transplantation: a prospective study. Bone Marrow Transplant. 2000;26:327–332. doi: 10.1038/sj.bmt.1702504. [DOI] [PubMed] [Google Scholar]
- 2.Uzunel M, Jaksch M, Mattsson J, Ringden O. Minimal residual disease detection after allogeneic stem cell transplantation is correlated to relapse in patients with acute lymphoblastic leukaemia. Br J Haematol. 2003;122:788–794. doi: 10.1046/j.1365-2141.2003.04495.x. [DOI] [PubMed] [Google Scholar]
- 3.Khan F, Agarwal A, Agrawal S. Significance of chimerism in hematopoietic stem cell transplantation: new variations on an old theme. Bone Marrow Transplant. 2004;34:1–12. doi: 10.1038/sj.bmt.1704525. [DOI] [PubMed] [Google Scholar]
- 4.Thiede C. Diagnostic chimerism analysis after allogeneic stem cell transplantation: new methods and markers. Am J Pharmacogenomics. 2004;4:177–187. doi: 10.2165/00129785-200404030-00005. [DOI] [PubMed] [Google Scholar]
- 5.Oliver DH, Thompson RE, Griffin CA, Eshleman JR. Use of single nucleotide polymorphisms (SNP) and real-time polymerase chain reaction for bone marrow engraftment analysis. J Mol Diagn. 2000;2:202–208. doi: 10.1016/S1525-1578(10)60638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alizadeh M, Bernard M, Danic B, Dauriac C, Birebent B, Lapart C, Lamy T, Le Prise PY, Beauplet A, Bories D, Semana G, Quelvennec E. Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real-time quantitative polymerase chain reaction. Blood. 2002;99:4618–4625. doi: 10.1182/blood.v99.12.4618. [DOI] [PubMed] [Google Scholar]
- 7.Sachidanandam SR, Weissman D, Schmidt SC, Kakol JM, Stein LD, Marth G, Sherry S, Mullikin JC, Mortimore BJ, Willey DL, Hunt SE, Cole CG, Coggill PC, Rice CM, Ning Z, Rogers J, Bentley DR, Kwok PY, Mardis ER, Yeh RT, Schultz B, Cook L, Davenport R, Dante M, Fulton L, Hillier L, Waterston RH, McPherson JD, Gilman B, Schaffner S, Van Etten WJ, Reich D, Higgins J, Daly MJ, Blumenstiel B, Baldwin J, Stange-Thomann N, Zody MC, Linton L, Lander ES, Altshuler D. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature. 2001;409:928–933. doi: 10.1038/35057149. [DOI] [PubMed] [Google Scholar]
- 8.Maas F, Schaap N, Kolen S, Zoetbrood A, Buno I, Dolstra H, de Witte T, Schattenberg A, van de Wiel-van Kemenade E. Quantification of donor and recipient hemopoietic cells by real-time PCR of single nucleotide polymorphisms. Leukemia. 2003;17:621–629. doi: 10.1038/sj.leu.2402856. [DOI] [PubMed] [Google Scholar]
- 9.Newton CR, Graham A, Heptinstall LE, Powell SJ, Summers C, Kalsheker N, Smith JC, Markham AF. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS) Nucleic Acids Res. 1989;17:2503–2516. doi: 10.1093/nar/17.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newton CR, Heptinstall LE, Summers C, Super M, Schwarz M, Anwar R, Graham A, Smith JC, Markham AF. Amplification refractory mutation system for prenatal diagnosis and carrier assessment in cystic fibrosis. Lancet. 1989;2:1481–1483. doi: 10.1016/s0140-6736(89)92931-0. [DOI] [PubMed] [Google Scholar]
- 11.Harries LW, Wickham CL, Evans JC, Rule SA, Joyner MV, Ellard S. Analysis of haematopoietic chimaerism by quantitative real-time polymerase chain reaction. Bone Marrow Transplant. 2005;35:283–290. doi: 10.1038/sj.bmt.1704764. [DOI] [PubMed] [Google Scholar]
- 12.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 13.Dewitte K, Fierens C, Stockl D, Thienpont LM. Application of the Bland-Altman plot for interpretation of method-comparison studies: a critical investigation of its practice. Clin Chem. 2002;48:799–801. [PubMed] [Google Scholar]
- 14.Willasch A, Schneider G, Reincke BS, Shayegi N, Kreyenberg H, Kuci S, Weber G, Van Der Reijden B, Niethammer D, Klingebiel T, Bader P. Sequence polymorphism systems for quantitative real-time polymerase chain reaction to characterize hematopoietic chimerism-high informativity and sensitivity as well as excellent reproducibility and precision of measurement. Lab Hematol. 2007;13:73–84. doi: 10.1532/LH96.07004. [DOI] [PubMed] [Google Scholar]
- 15.Chalandon Y, Vischer S, Helg C, Chapuis B, Roosnek E. Quantitative analysis of chimerism after allogeneic stem cell transplantation by PCR amplification of microsatellite markers and capillary electrophoresis with fluorescence detection: the Geneva experience. Leukemia. 2003;17:228–231. doi: 10.1038/sj.leu.2402758. [DOI] [PubMed] [Google Scholar]
- 16.Hancock JP, Goulden NJ, Oakhill A, Steward CG. Quantitative analysis of chimerism after allogeneic bone marrow transplantation using immunomagnetic selection and fluorescent microsatellite PCR. Leukemia. 2003;17:247–251. doi: 10.1038/sj.leu.2402759. [DOI] [PubMed] [Google Scholar]
- 17.Kreyenberg H, Holle W, Mohrle S, Niethammer D, Bader P. Quantitative analysis of chimerism after allogeneic stem cell transplantation by PCR amplification of microsatellite markers and capillary electrophoresis with fluorescence detection: the Tuebingen experience. Leukemia. 2003;17:237–240. doi: 10.1038/sj.leu.2402761. [DOI] [PubMed] [Google Scholar]
- 18.Schraml E, Daxberger H, Watzinger F, Lion T. Quantitative analysis of chimerism after allogeneic stem cell transplantation by PCR amplification of microsatellite markers and capillary electrophoresis with fluorescence detection: the Vienna experience. Leukemia. 2003;17:224–227. doi: 10.1038/sj.leu.2402756. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg EP, Miklos DB, Neuberg D, Eichner DA, McLaughlin SF, Mattes-Ritz A, Alyea EP, Antin JH, Soiffer RJ, Ritz J. A novel rapid single nucleotide polymorphism (SNP)-based method for assessment of hematopoietic chimerism after allogeneic stem cell transplantation. Blood. 2003;101:363–369. doi: 10.1182/blood-2002-05-1365. [DOI] [PubMed] [Google Scholar]
- 20.Fredriksson M, Barbany G, Liljedahl U, Hermanson M, Kataja M, Syvanen AC. Assessing hematopoietic chimerism after allogeneic stem cell transplantation by multiplexed SNP genotyping using microarrays and quantitative analysis of SNP alleles. Leukemia. 2004;18:255–266. doi: 10.1038/sj.leu.2403213. [DOI] [PubMed] [Google Scholar]
- 21.Eshel R, Vainas O, Shpringer M, Naparstek E. Highly sensitive patient-specific real-time PCR SNP assay for chimerism monitoring after allogeneic stem cell transplantation. Lab Hematol. 2006;12:39–46. doi: 10.1532/LH96.05034. [DOI] [PubMed] [Google Scholar]
- 22.Jimenez-Velasco A, Barrios M, Roman-Gomez J, Navarro G, Buno I, Castillejo JA, Rodriguez AI, Garcia-Gemar G, Torres A, Heiniger AI. Reliable quantification of hematopoietic chimerism after allogeneic transplantation for acute leukemia using amplification by real-time PCR of null alleles and insertion/deletion polymorphisms. Leukemia. 2005;19:336–343. doi: 10.1038/sj.leu.2403622. [DOI] [PubMed] [Google Scholar]
- 23.Ayyadevara S, Thaden JJ, Shmookler Reis RJ. Discrimination of primer 3′-nucleotide mismatch by taq DNA polymerase during polymerase chain reaction. Anal Biochem. 2000;284:11–18. doi: 10.1006/abio.2000.4635. [DOI] [PubMed] [Google Scholar]
- 24.Liang P, Pardee AB. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.