Abstract
Objective
To determine the respective roles of socio-economic status (SES) and ethnicity in the risk of incident metabolic syndrome in middle-aged women.
Design and participants
A total of 3302 pre- and peri-menopausal women, not receiving hormone therapy at baseline, took part in the Study of Women’s Health Across the Nation, a multi-site, community-based, longitudinal study of the menopausal transition. The main outcome measures were to ascertain the prevalence of the metabolic syndrome and the incidence of the metabolic syndrome over 5 years of follow-up.
Results
At baseline, the prevalence of the metabolic syndrome was 21% (n = 673). Among 2512 women without metabolic syndrome at baseline, 12.8% (n = 321) developed the metabolic syndrome during 5 years of follow-up. Both ethnicity and SES were significant univariate predictors of incident metabolic syndrome. In multivariate logistic regression models that included age at baseline, menopausal status and site, baseline smoking and alcohol consumption at follow-up visit 1, as well as baseline values of each of the components of the metabolic syndrome, only education was an independent predictor of incident metabolic syndrome.
Conclusion
Approximately 13% of peri-menopausal women developed the metabolic syndrome during the 5-year follow-up period. Education, but not ethnicity, was an independent predictor of incident metabolic syndrome risk.
Keywords: ethnicity, longitudinal studies, metabolic syndrome, socio-economic status, women
Introduction
The clustering of obesity, dyslipidaemia, hypertension and impaired glucose metabolism has been defined as the metabolic syndrome [1–3]. The metabolic syndrome is associated with accelerated arterial ageing [4] and is highly prevalent in older subjects, in whom it is an independent predictor of cardiovascular (CV) events [5].
Women usually develop CV disease later in life than men [6,7] with a steeper increase after the age of 50 and this has been attributed to the loss of female sex hormones at the time of menopause [6,7]. Middle-age women experience increases in waist girth, plasma lipids, blood pressure and impaired glucose metabolism [8,9], all components of the metabolic syndrome. Ethnic differences in insulin sensitivity, body mass index and other CV risk factors have been reported in middle-aged women [10,11]. Furthermore, SES has also been recognized as an important predictor of CV disease, both in men and women [12]. Whether ethnicity and socio-economic status (SES) interact to influence the risk of incident metabolic syndrome is yet unknown.
The aim of the present study was to evaluate whether SES and ethnicity were independent predictors of the metabolic syndrome in middle-age women. We addressed this aim in the Study of Women’s Health Across the Nation (SWAN) a multi-site, multi-ethnic longitudinal observational study designed to examine the physical, psychological and social changes that women experience during the menopausal transition. We first examined the prevalence of the metabolic syndrome in SWAN participants at baseline according to their ethnicity, income and educational level. Then we evaluated the incidence of the metabolic syndrome according to SES and ethnicity among those who were not classified as having the metabolic syndrome at baseline. Any observed associations between SES and ethnicity with incident metabolic syndrome could be simply because of their associations with elevated baseline levels of the individual risk factors that comprise the metabolic syndrome. To assess whether SES and ethnicity have any predictive value beyond these individual components, we repeated the longitudinal analyses of incident metabolic syndrome, taking into account the baseline levels of the metabolic syndrome components. We also examined potential interactions between SES and ethnicity in relation to metabolic syndrome.
Participants and methods
At baseline, SWAN recruited a total of 3302 women between the ages of 42 and 52 from seven sites: Boston, MA; Chicago, IL; Detroit, IL; Los Angeles, CA; Newark, NJ; Pittsburgh, PA; and Oakland, CA.
The design and sampling technique of SWAN have been provided in detail [13]. Briefly, in order to be eligible for the study, women were required to have an intact uterus, at least one menstrual period and no use of reproductive hormones in the previous 3 months. All seven sites enrolled non-Hispanic whites and each site also enrolled women belonging to one pre-specified minority ethnic group: African-American women at Boston, Chicago, Detroit and Pittsburgh; Japanese women at Los Angeles; Chinese women at Oakland; and Hispanic women at Newark. Specifically, the racial distribution of our study population consisted of 1550 (46.9%) Caucasian, 250 (7.6%) Chinese, 281 (8.5%) Japanese, 286 (8.7%) Hispanic and 935 (28.3%) African-American middle-aged women.
The current analyses used available data from the baseline visit and the 5-year follow-up visit. Women with metabolic syndrome at baseline were excluded from the longitudinal analyses.
Questionnaires and physical measurements
Demographic information, smoking status, level of alcohol consumption, ethnicity, hormone therapy use, SES and menopausal status were obtained using annually administered standardized questionnaires. Height was measured using a stadiometer or portable scale; weight was measured using a balance beam, digital scale or portable scale. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. With the exception of SES (education) and alcohol consumption, the former was only collected at baseline and the latter was obtained at follow-ups 1 to 4; all of the aforementioned variables were collected at baseline and on all four follow-up visits.
In SWAN, menopausal status was classified as a categorical variable at each clinic visit. Pre-menopause was defined as having a menstrual period in the past 3 months with no change in cycle regularity in the past 12 months. Early peri-menopause was defined as having a menstrual period in the past 3 months with some change in cycle regularity in the past 12 months. Late peri-menopause was defined as no menstrual period in the past 3 months but some menstrual bleeding within the past 12 months. Post-menopause was defined as having no menstrual period within the past 12 months.
Socio-economic status
SES was measured at baseline by three variables—education, self-reported ability to pay for basic needs and family income. For brevity, only education and family income were used for the purposes of the present study. Education consisted of five categories—less than high school, high school graduate/general educational development (GED) equivalent, some college, college graduate, post-college education (reference group). Yearly income consisted of five categories—less than $20 000, $20 000–34 999, $35 000–49 999, $50 000–74 999 and greater than $75 000 (reference group).
Measurements of components of the metabolic syndrome
Blood samples were collected on days 2–5 of the follicular phase in 86.7% of women and after a 12-h fast in 96.2% of all women. Serum insulin level was measured using a solid-phase radioimmunoassay (DPC Coat-A-Count Insulin RIA; Diagnostic Products, Los Angeles, CA, USA). All lipids were analysed on EDTA-treated plasma. Triglycerides were analysed by enzymatic methods using a Hitachi 747 analyser (Boehringer Mannheim Diagnostics, Indianapolis, IN, USA). High-density lipoprotein (HDL) cholesterol was isolated using heparin-2M manganese chloride. Glucose levels were measured using a hexokinase-coupled reaction (Boehringer Mannheim Diagnostics). Low-density lipoprotein cholesterol (LDL) was calculated using the Friedwald equation.
Two blood pressure measurements were obtained on the right arm after 5 min of rest in a seated position and averaged. Waist circumference was measured over undergarments or light clothing at the narrowest part of the waist.
All these measurements were performed at each visit; assays were performed at all visits with the exception of year 2.
Definition of the metabolic syndrome
Participants who satisfied at least three of the following five criteria were considered to have the metabolic syndrome: abdominal obesity, high triglycerides, low HDL cholesterol, high blood pressure and impaired fasting glucose or diabetes [1]. Abdominal obesity was defined as a waist circumference greater than or equal to 80 cm for Chinese and Japanese women and a waist circumference greater than or equal to 88 cm for Caucasian, Hispanic and African-American women [14]. Hypertriglyceridaemia was defined as triglycerides greater than or equal to 150 mg/dl. Low HDL was defined as an HDL cholesterol level less than 50 mg/dl. High blood pressure was defined as systolic blood pressure greater than or equal to 130 mmHg or diastolic blood pressure greater than or equal to 85 mmHg or current blood pressure medication use. Impaired fasting glucose was defined as fasting blood glucose levels greater than or equal to 110 mg/dl or classification as diabetic. The metabolic syndrome variable was available at all four visits.
Statistical analyses
Clinical characteristics of participants without metabolic syndrome at baseline were compared across the individual SES variables (income and education) at baseline or ethnicity using standard χ2-tests or one-way ANOVAs as appropriate. Further descriptive statistics are presented for site by testing difference in proportions for income and education.
Univariate and multivariate logistic regression analysis were used to predict the cumulative incidence of the metabolic syndrome over 5 years of follow-up. Because of lack of model fit for baseline income as assessed by Hosmer and Lemeshow goodness-of-fit test in logistic regression models, we decided to focus on education as the sole indicator of SES in all subsequent analyses. For the multivariate logistic regression models, the model building strategy was to start with a base model that included site, menopause status and age at baseline as independent variables, then to evaluate the impact of education and ethnicity by adding them individually to this base model. As a next step, we controlled for the individual components of the metabolic syndrome at baseline and smoking status at baseline and alcohol consumption (moderate consumers vs. light consumers and non-consumers who drank less than one alcoholic beverage a month) at visit 1, and again evaluated the influence of education and ethnicity by adding them individually to the fully adjusted model. Interaction terms between education and ethnicity were evaluated to determine whether the association between education and metabolic syndrome was influenced by ethnicity. Likelihood ratio tests were used to assess whether individual variables significantly contributed to the prediction of metabolic syndrome. Model fit was verified using the Hosmer and Lemeshow goodness-of-fit test.
Analyses were carried out using SAS version for Windows 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
Prevalence of the metabolic syndrome in middle-age women
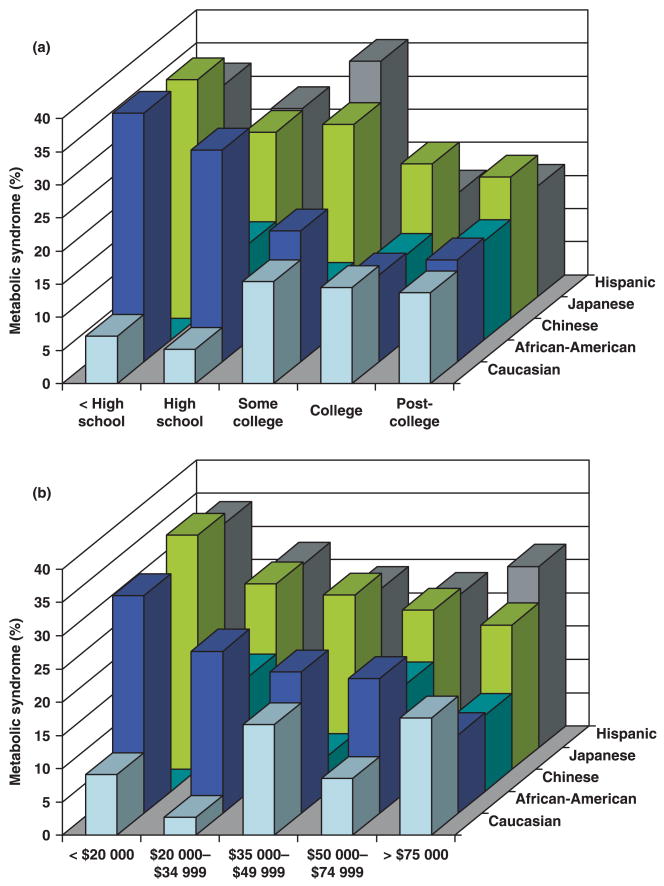
At baseline, the prevalence of the metabolic syndrome in our study cohort was 21.1% (n = 673). Figure 1 shows the prevalence of the metabolic syndrome stratified according to SES (education in part A and income part B) and ethnicity. Prevalent metabolic syndrome and its component risk factors were associated with the indicators of socio-economic disadvantage (see Table 1 for details on education and Table 2 for details on income). The lower the SES, the higher the prevalence of the metabolic syndrome. The prevalence of metabolic syndrome was 14.2% in women with an annual income greater than $75 000 and 33.0% in women with an annual income less than $20 000 and differed among the five income groups (P < 0.0001). The prevalence of metabolic syndrome was 16.5% in women with a post-college educational degree and 30.0% in women with less than a high school degree and varied among the five education categories (P < 0.0001). Significant ethnic differences in the prevalence of the metabolic syndrome among middle-age women were also observed: 19.1% in Caucasian, 12.1% in Chinese, 11.9% in Japanese, 30.3% in Hispanic and 27.1% in African-American women (P < 0.0001).
FIGURE 1.
Prevalence of the metabolic syndrome in middle-age women by socio-economic status and ethnicity. (a) Prevalence of Metabolic Syndrome by Education and Ethnicity; (b) Prevalence of Metabolic Syndrome by Income and Ethnicity.
Table 1.
Baseline levels of risk factors comprising the metabolic syndrome according to educational status in women without the metabolic syndrome at baseline
| < High school
(n = 156) |
High school graduate
(n = 411) |
Some college
(n = 784) |
College graduate
(n = 533) |
Post-college
(n = 607) |
Overall P-value | |
|---|---|---|---|---|---|---|
| Age (years) | 46.5 ± 2.7 | 46.3 ± 2.7 | 46.1 ± 2.7 | 46.0 ± 2.6 | 46.6 ± 2.6 | < 0.01 |
| Waist circumference (cm) | 85.2 ± 14.0 | 83.4 ± 13.9 | 82.8 ± 14.5 | 80.0 ± 13.2 | 80.2 ± 11.3 | < 0.01 |
| HDL cholesterol (mg/dl) | 57.9 ± 14.5 | 57.9 ± 14.9 | 59.3 ± 13.8 | 60.3 ± 13.1 | 61.0 ± 13.2 | < 0.01 |
| SBP (mmHg) | 120.6 ± 16.6 | 116.3 ± 15.3 | 115.7 ± 16.0 | 112.2 ± 13.8 | 113.3 ± 15.2 | < 0.01 |
| DBP (mmHg) | 77.1 ± 10.1 | 74.1 ± 10.7 | 74.2 ± 10.3 | 73.2 ± 9.1 | 73.1 ± 9.7 | < 0.01 |
| Fasting glucose (mg/dl) | 98.0 ± 32.8 | 92.2 ± 15.7 | 91.9 ± 15.2 | 90.5 ± 11.5 | 91.0 ± 12.3 | < 0.01 |
| Triglycerides (mg/dl) | 100.5 ± 48.1 | 95.5 ± 45.2 | 91.9 ± 42.8 | 90.3 ± 38.5 | 87.7 ± 56.2 | < 0.05 |
| High triglycerides (%) | 9.9 | 8.9 | 7.0 | 6.4 | 5.5 | 0.16 |
| High BP (%) | 28.2 | 21.8 | 22.4 | 17.6 | 17.5 | < 0.01 |
| Impaired glucose (%) | 7.3 | 4.7 | 3.3 | 2.1 | 2.5 | < 0.01 |
| Low HDL (%) | 25.0 | 27.5 | 23.0 | 19.2 | 17.7 | < 0.01 |
| Abdominal obesity (%) | 31.6 | 29.5 | 30.9 | 22.4 | 20.9 | < 0.01 |
Means and SDs are provided for the first seven measures in the table, percentages for the remaining five measures.
High triglycerides: triglycerides ≥ 150 mg/dl.
High BP: BP ≥ 130 mmHg or DBP ≥ 85 mmHg or current blood pressure medication use.
Impaired glucose: fasting blood glucose ≥ 110 mg/dl or classification as diabetic.
Low HDL: HDL cholesterol level < 50 mg/dl.
Abdominal obesity: waist circumference ≥ 80 cm for Chinese and Japanese women, ≥ 88 cm for Caucasian, Hispanic and African-American women.
Varying amount of missing data on each variable; complete data on age.
BP, blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; SBP, systolic blood pressure; SD, standard deviation.
Table 2.
Baseline levels of risk factors comprising the metabolic syndrome according to income group in women without the metabolic syndrome at baseline
| < $20 000
(n = 310) |
$20 000–34 999
(n = 370) |
$35 000– 49 999
(n = 435) |
$50 000–74 999
(n = 576) |
> $75 000
(n = 749) |
Overall P-value | |
|---|---|---|---|---|---|---|
| Age (years) | 46.0 ± 2.7 | 46.1 ± 2.6 | 46.2 ± 2.6 | 46.2 ± 2.6 | 46.5 ± 2.7 | < 0.05 |
| Waist circumference (cm) | 87.9 ± 16.8 | 83.0 ± 13.2 | 82.3 ± 14.3 | 81.1 ± 12.5 | 78.7 ± 10.9 | < 0.01 |
| HDL cholesterol (mg/dl) | 57.5 ± 14.6 | 58.8 ± 13.4 | 59.0 ± 14.0 | 59.2 ± 12.7 | 61.3 ± 13.9 | < 0.01 |
| SBP (mmHg) | 120.0 ± 17.6 | 116.5 ± 16.1 | 113.8 ± 14.7 | 113.5 ± 15.3 | 113.1 ± 13.8 | < 0.01 |
| DBP (mmHg) | 76.5 ± 10.7 | 74.7 ± 10.3 | 73.1 ± 9.9 | 73.2 ± 9.9 | 73.5 ± 9.2 | < 0.01 |
| Fasting glucose (mg/dl) | 94.0 ± 20.2 | 92.7 ± 18.8 | 90.6 ± 12.8 | 91.6 ± 12.5 | 91.3 ± 15.0 | < 0.05 |
| Triglycerides (mg/dl) | 98.7 ± 46.5 | 94.5 ± 66.0 | 89.4 ± 40.5 | 91.7 ± 44.1 | 88.7 ± 39.9 | < 0.05 |
| High triglycerides (%) | 7.6 | 7.5 | 5.2 | 7.4 | 7.1 | 0.63 |
| High BP (%) | 24.8 | 23.5 | 18.5 | 19.9 | 17.9 | < 0.05 |
| Impaired glucose (%) | 4.0 | 4.7 | 2.4 | 3.0 | 2.9 | 0.33 |
| Low HDL (%) | 24.5 | 24.9 | 22.5 | 21.9 | 18.6 | 0.09 |
| Abdominal obesity (%) | 39.3 | 32.6 | 26.8 | 24.5 | 19.2 | < 0.01 |
Means and SDs are provided for the first seven measures in the table, percentages for the remaining five measures.
High triglycerides: triglycerides ≥ 150 mg/dl.
High BP: SBP ≥ 130 mmHg or DBP ≥ 85 mmHg, or current blood pressure medication use.
Impaired glucose: fasting blood glucose ≥ 110 mg/dl or classification as diabetic.
Low HDL: HDL cholesterol level < 50 mg/dl.
Abdominal obesity: waist circumference ≥ 80 cm for Chinese and Japanese women, ≥ 88 cm for Caucasian, Hispanic and African-American women.
Varying amount of missing data on each variable; complete data on age.
BP, blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; SBP, systolic blood pressure; SD, standard deviation.
When testing for an interaction between SES and ethnicity, we observed a trend for difference among ethnic groups in the prevalence of the metabolic syndrome with changing SES (P = 0.06).
Predictors of the 5-year incidence of the metabolic syndrome
Among 2512 women without metabolic syndrome at baseline, 13.9% were missing as a result of a lack of information on individual components and 17.8% were lost to follow-up. Participants with a missing diagnosis did not differ from participants remaining in the analysis in education level (P = 0.44) and income (P = 0.21), but differed in age (P = 0.0002; 45.8 years for those missing diagnosis and 46.4 years for those remaining in the analysis). Participants who were lost to follow-up differed from participants remaining in the analysis in income [P < 0.0001; higher proportion lost to follow-up for low income (33.9, 26.7, 18.5 and 15.4% for less than $20 000, $20 000–34 999, $35 000–49 999, $50 000–74 999 and greater than $75 000, respectively)], education level (P < 0.0001; higher proportion lost to follow-up from lowest education level: 30.2, 22.1, 15.6 and 14.3% some college, college graduate and post-college education, respectively) and age (P < 0.0001; 45.9 years for those missing data and 46.3 years for those remaining in the analysis).
Over the 5 years of follow-up period, 12.8% (n = 321) women developed the metabolic syndrome.
In unadjusted analyses, each of the two SES variables was associated with incident metabolic syndrome at 5 years (P < 0.0001). Specifically, middle-age women with high school degree or less education showed a significantly higher risk of developing the metabolic syndrome as compared with middle-age women with at least a post-college degree [odds ratio (OR) 3.46, 95% confidence interval (CI) 2.03–5.89]; middle-age women with an annual income less than $20 000 showed a significantly higher risk of developing the metabolic syndrome as compared with middle-age women with an annual income > $75 000 (OR 3.13, 95% CI 2.10–4.66).
In univariate analyses, ethnicity was also associated with incident metabolic syndrome across 5 years of follow-up (P < 0.0001). Compared with Caucasians, Japanese women had a significantly lower risk (OR 0.64, 95% CI 0.40–1.03) and Chinese women a non-significantly lower risk (OR 0.71, 95% CI 0.43–1.16) of developing the metabolic syndrome, whereas Hispanic (OR 3.77, 95% CI 2.32–6.13) and African-American women (OR 1.25, 95% CI 1.01–1.56) showed a significantly higher risk of developing the metabolic syndrome over 5 years of follow-up.
In the base model evaluating incident metabolic syndrome, only site was a significant predictor (P < 0.0001), whereas menopausal status and baseline age were not. Education (P < 0.0001) and ethnicity (P = 0.01) were also significant predictors of incident metabolic syndrome when they were added individually to the base model.
As shown elsewhere in SWAN women [13,15], a more unfavourable CV risk profile at baseline was observed in women with lower as compared with those with higher SES. We sought to investigate whether the role of ethnicity and/or SES in predicting incident metabolic syndrome was simply a reflection of differences in the baseline values of the components of the metabolic syndrome (i.e. blood pressure, glucose, lipids, obesity) or whether ethnicity and/or SES provided prognostic information about the risk of developing the metabolic syndrome in middle-age women, independent of levels of those components. Additional multivariate logistic regression models were therefore constructed that included the baseline values of each of the components of the metabolic syndrome in addition to study site, menopausal status and age at baseline. Data on ethnicity and education are illustrated in Table 3.
Table 3.
Predictors of cumulative incident metabolic syndrome in middle-age women over 5 years of follow-up
| Cumulative incidence of metabolic syndrome
|
||||
|---|---|---|---|---|
| Model 1* |
Model 2* |
|||
| Ethnicity | OR | 95% CI | OR | 95% CI |
| African-American | 1.07 | 0.80–1.44 | 0.91 | 0.61–1.35 |
| Hispanic | 1.43 | 0.48– 4.29 | 0.50 | 0.11–2.19 |
| Japanese | 1.01 | 0.51–1.98 | 0.99 | 0.44–2.63 |
| Chinese | 0.70 | 0.35–1.31 | 1.17 | 0.50–2.75 |
| Caucasian | 1.00 | — | 1.00 | — |
|
| ||||
| Model 1†
|
Model 2†
|
|||
| Education | OR | 95% CI | OR | 95% CI |
|
| ||||
| High school or less | 1.46 | 1.16– 1.84 | 1.38 | 1.02–1.85 |
| Some college | 1.25 | 1.02–1.53 | 0.99 | 0.76–1.28 |
| College graduate | 0.84 | 0.66–1.07 | 0.94 | 0.69–1.28 |
| Post-college | 1.00 | — | 1.00 | — |
CI, confidence interval; OR, odds ratio.
Models, *ethnicity and †education.
Model 1 adjusts for site, menopausal status and age at baseline.
Model 2 adjusts for site, menopausal status and age at baseline, baseline smoking, alcohol consumption at visit 1 and baseline levels of individual components of the metabolic syndrome (waist, triglycerides, high-density lipoprotein, blood pressure, and fasting glucose).
Reference group is Caucasian for ethnicity and post-college for education.
In all the statistical models entertained, site was once again an important predictor of incident metabolic syndrome (P < 0.03), whereas age at baseline and menopausal status were not. Findings about the impact of site on the development of metabolic syndrome over the 5-year follow-up period are presented in Table 4. Participants in Newark showed a substantial increased risk (P < 0.05) compared with the participants living in Pittsburgh. Furthermore, women in Detroit, Chicago and Pittsburgh were twofold more likely to develop metabolic syndrome when compared with women from Boston (P < 0.05). Women from Oakland show a twofold increased risk when compared with women from Boston (P < 0.05) only in the model evaluating the impact of education but not ethnicity. Baseline levels of each of the components of the metabolic syndrome were significantly associated with the risk of developing the metabolic syndrome over the 5-year follow-up period (all P < 0.0001). When ethnicity was added to these models it was no longer a predictor of incident metabolic syndrome (P = 0.37; see Table 3 for details on ORs). Conversely, when education was added to these models which were adjusted for baseline values of the individual components, it remained an independent predictor of incident metabolic syndrome (P = 0.003; refer to Table 3 for details on ORs). When both ethnicity and education and their interaction were added to the multivariate logistic model, the interaction term was not significant (P = 0.73). Baseline smoking was not a statistically significant predictor (P > 0.15) of cumulative incidence of metabolic syndrome; with an 11 and 12% increased risk for smokers in the ethnicity model and education model, respectively. Likewise, alcohol consumption at follow-up visit 1 was not a statistically significant predictor (P > 0.75) of cumulative incidence of metabolic syndrome; with a 1 or 3% increased risk for alcohol consumers in the ethnicity and education model, respectively.
Table 4.
Impact of site on the predictions of cumulative incident metabolic syndrome in middle-age women by ethnicity and education over 5 years of follow-up
| Model | Statistics | Detroit | Boston | Chicago | Oakland | Los Angeles | Newark | Pittsburgh |
|---|---|---|---|---|---|---|---|---|
| Ethnicity | OR | 1.40 | 0.58 | 1.42 | 1.28 | 1.00 | 4.78* | 1.00 |
| 95% CI | 0.77–2.53 | 0.30–1.11 | 0.79–2.53 | 0.61–2.71 | 0.47–2.13 | 1.13–20.15 | — | |
| Education | OR | 1.39 | 0.61 | 1.54 | 1.39 | 0.98 | 2.07* | 1.00 |
| 95% CI | 0.65–1.25 | 0.32–1.17 | 0.86–2.75 | 0.77–2.51 | 0.53–1.80 | 1.02–4.22 | — |
CI, confidence interval; OR, odds ratio.
Model adjusts for site, menopausal status and age at baseline, baseline smoking, alcohol consumption at visit 1 and baseline levels of individual components of the metabolic syndrome (waist, triglycerides, high-density lipoprotein, blood pressure, and fasting glucose); reference site is Pittsburgh.
Effects of SES and ethnicity on distribution of altered components of the metabolic syndrome in unaffected women at baseline
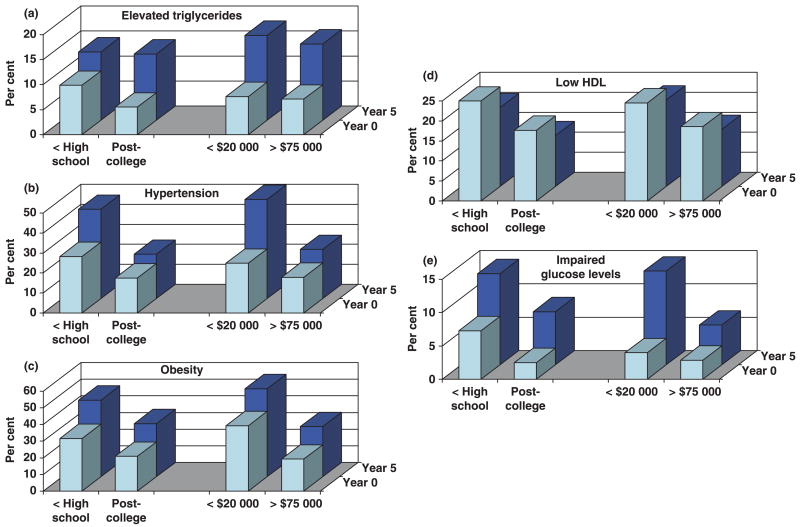
As shown in Fig. 2, middle-age women with lower SES showed a higher prevalence of each component of the metabolic syndrome at baseline than women with high SES. Irrespective of SES status, each component shows a worse profile at follow-up 5 compared with baseline. The respective relative increase over a 5-year follow-up did not vary significantly with SES, with the exception of elevated glucose (P = 0.01) and hypertension (P = 0.0001), which showed a steeper increase over time in women with lower income.
FIGURE 2.
Distribution of altered components of the metabolic syndrome by socio-economic status after 5 years of follow-up. HDL, high-density lipoprotein.
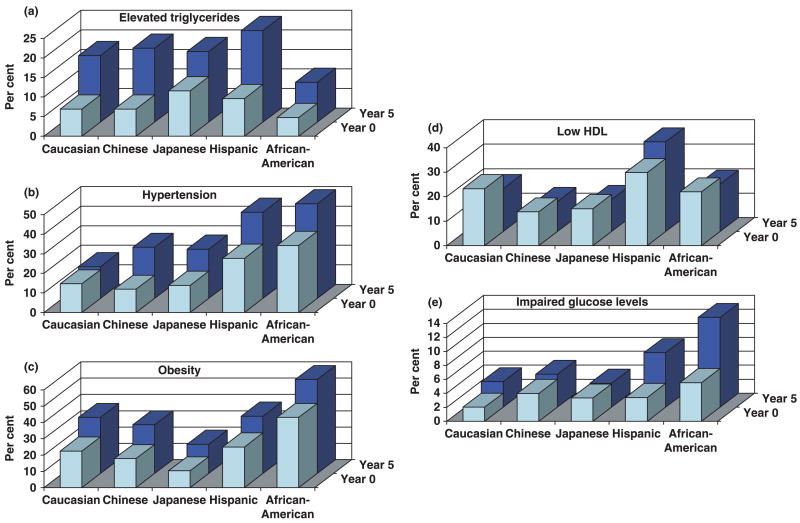
As shown in Fig. 3, the prevalence of each component of the metabolic syndrome at baseline differed by ethnicity, with African-American women showing the highest prevalence of abdominal obesity, impaired glucose level and elevated blood pressure and Hispanic women showing the highest prevalence of elevated glucose, triglyceride levels and lower HDL cholesterol. The respective relative increase over a 5-year follow-up did not vary significantly with SES, with the exception of elevated glucose (a steeper increase over time in African-American women; P = 0.01) and low HDL cholesterol (an increase in Hispanic women compared with a decrease in other ethnic groups).
FIGURE 3.
Distribution of altered components of the metabolic syndrome by ethnicity after 5 years of follow-up. HDL, high-density lipoprotein.
Discussion
The prevalence of the metabolic syndrome in our population was 21%. This is similar to the findings of the Third National Health and Nutrition Examination Survey (NHANES III), which reported a prevalence of the metabolic syndrome of 23.3% in women aged 40–59, or a prevalence of 21.2% in the same groups when subjects with diabetes mellitus were excluded [16]. Our findings on the increased prevalence of the metabolic syndrome in lower SES groups are consistent with previous cross-sectional studies. In the NHANES III cross-sectional study, in addition to post-menopausal status and Mexican-American ethnicity, lower income was associated with increased odds of the metabolic syndrome [17]. The Whitehall II study also described, cross-sectionally, an inverse significant relationship between SES and central obesity and other components of the metabolic syndrome [18]. Conversely, another cross-sectional study failed to show any significant effect of SES on metabolic syndrome components in African-Americans [19]. An earlier report from the Whitehall II study suggested that health-related behaviours, such as smoking, alcohol consumption and physical activity, accounted only for a part of the social patterning of prevalent metabolic syndrome [18]. In contrast, our findings did not show a significant prediction of metabolic syndrome by smoking or alcohol consumption beyond other risk factors in our sample. In the SWAN women, ethnicity affected the 10-year risk of myocardial infarction or coronary death derived from the Framingham risk equation and other factors contributing to the metabolic syndrome [13]. This study also showed that adjustments for education accounted for a significant part of the ethnic differences [13].
Metabolic syndrome prevalence varied by SES (the lower the SES, the higher the prevalence of the metabolic syndrome) and ethnicity (the highest in Hispanic and African-American women, the lowest in Chinese and Japanese women). Approximately 10% of SWAN cohort middle-age women developed the metabolic syndrome during 5 years of follow-up. Both ethnicity and education were significant univariate predictors of cumulative incident metabolic syndrome. However, when controlling for age, menopausal status and baseline levels of factors contributing to the metabolic syndrome, only education but not ethnicity was an independent predictor of incident metabolic syndrome.
To the best of our knowledge, this is the first longitudinal study investigating the roles of SES and ethnicity simultaneously measured on development of the metabolic syndrome in middle-aged women. In the present study, we observed that approximately 10% of women developed the metabolic syndrome over a 5-year follow-up period. Both lower SES and ethnicity, specifically African-American and Hispanic ethnicity, were associated with increased risk of developing the metabolic syndrome in middle-age women. However, after accounting for baseline levels of the components of the metabolic syndrome, only low educational attainment, but not income or ethnicity, persisted as an independent predictor of incident metabolic syndrome. Thus, it is possible that the increased risk of incident metabolic syndrome associated with income or ethnicity was mediated via differences in the baseline values of the metabolic syndrome components. With specific regards to income, we cannot rule out that its lack of significance may be at least partly because of the SWAN categorization of income or to regional variation in the meaning of income (i.e. life cost differs between different regions of the country).
Why might educational attainment be a better SES predictor of the development of the metabolic syndrome than income? Some suggest that education is a better marker of SES because educational attainment is often completed in young adulthood and is less contaminated by poor health, which typically emerges in midlife. Others note that education may be a better SES marker for women because women may not be in the labour force or perhaps occupy jobs of less status, with the same credentials relative to men [20]. Nonetheless, it should be noted that SES is a multidimensional construct and can be measured by other markers, such as neighbourhood-level SES, subjective SES and childhood SES [21]. Our paper only examined a few indicators of SES.
Our results showing low educational attainment as an important predictor of incident metabolic syndrome in middle-age women is consistent with previous research showing that educational attainment was inversely associated with levels of CV risk [22,23] or with subclinical vascular lesions [24,25] and that this association was not explained by conventional, non-dietary lifestyle factors [23]. An observation in Finland also showed that low education was associated with accelerated progression of vascular ageing and atherosclerosis over time [26]. Low education may be a marker for characteristics associated with increased risk for the metabolic syndrome, such as limited access to health care, higher personal and physical stress and differences in body image that influence motivation to lose weight. Indeed, high prevalence of overweight and obesity among black adults and in lower socio-economic groups has been reported in national surveys [27] and may play an important role in excess metabolic syndrome incidence in those subpopulations.
The longitudinal design, the large ethnic diversity and community-based population sample and the presence of variables related to SES are all major strengths of the present study. Potential limitations of our study are represented by the SWAN design, which included sampling of Japanese, Chinese and Hispanic women at one site each, confounding geography and ethnicity, whereas all seven study sites enrolled non-Hispanic Caucasian women and four sites enrolled African-Americans. Although it is possible that the women at a given site may not be representative of their ethnic group as a whole, we attempted to account for regional variation by adjusting for study site in all analyses.
A limitation of the study is that we had a differential loss to follow-up for the various SES groupings; higher proportions of low SES participants were lost to follow-up. Although the retention was not equal across the educational levels and income classes, we lost those who had higher values on each metabolic component to begin with and therefore would have been more likely to develop metabolic syndrome. Consequently, our finding regarding the impact of the SES as measured by education level could even be stronger if we had been able to retain in our study participants with low SES at the same rate as participants with high SES. It is possible that our findings that smoking or alcohol consumption does not significantly add to the prediction of the development of metabolic syndrome beyond other risk factors are as a result of the SES retention bias.
Our findings may have a potential substantial impact with respect to public health policy. In an era where the search for genetic factors predisposing to disease is attracting funding and attention, our study supports the relevance of improving population education to reduce the burden of the metabolic syndrome, a major risk factor for diabetes mellitus and CV disease. In the setting of primary care, increasing time and energy should be dedicated to motivate the subjects to increase the adherence to lifestyle modification, specifically weight loss and physical activity. Future studies are needed to individuate educational strategies to successfully reduce the burden of poor education on the risk of CV disease in women.
Acknowledgments
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH. We thank the study staff at each site and all the women who participated in SWAN.
Abbreviations
- CI
confidence interval
- CV
cardiovascular
- HDL
high-density lipoprotein
- OR
odds ratio
- SES
socioeconomic status
- SWAN
Study of Women’s Health Across the Nation
Footnotes
Competing interests
Nothing to declare.
Clinical centres
University of Michigan, Ann Arbor—MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA—Robert Neer, PI 1994–1999; Joel Finkelstein, PI 1999–present; Rush University, Rush University Medical Center, Chicago, IL—Lynda Powell, PI; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; University of Medicine and Dentistry—New Jersey Medical School, Newark—Gerson Weiss, PI 1994 –2004; Nanette Santoro, PI 2004–present; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI.
NIH programme office
National Institute on Ageing, Bethesda, MD—Marcia Ory 1994–2001; Sherry Sherman 1994 –present; National Institute of Nursing Research, Bethesda, MD—Program Officers.
Central laboratory
University of Michigan, Ann Arbor—Daniel McConnell; (Central Ligand Assay Satellite Services).
Coordinating centre
New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995–2001; University of Pittsburgh, Pittsburgh, PA—Kim Sutton-Tyrrell, PI 2001–present.
Steering committee
Chris Gallagher, Chair; Susan Johnson, Chair.
References
- 1.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation and treatment of high blood cholesterol in adults. J Am Med Assoc. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KGMM, Zimmet PZ for the WHO Consultation. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus, provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Meigs JB, D’Agostino RB, Sr, Wilson PW, Cupples LA, Nathan DM, Singer DE. Risk variable clustering in the insulin resistance syndrome. The Framingham Offspring Study. Diabetes. 1997;46:1594–600. doi: 10.2337/diacare.46.10.1594. [DOI] [PubMed] [Google Scholar]
- 4.Scuteri A, Najjar SS, Muller DC, Andres R, Hougaku H, Metter EJ, Lakatta EG. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol. 2004;43:1388–1395. doi: 10.1016/j.jacc.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 5.Scuteri A, Najjar SS, Morrell CH, Lakatta EG. The metabolic syndrome in older individuals: prevalence and prediction of cardiovascular events. The Cardiovascular Health Study. Diabetes Care. 2005;28:882–887. doi: 10.2337/diacare.28.4.882. [DOI] [PubMed] [Google Scholar]
- 6.Kannel WB, Hjortland MC, McNamara PM, Gordon T, Kannel WB, Hjortland MC, et al. Menopause and risk of cardiovascular disease: the Framingham Study. Ann Intern Med. 1978;85:447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 7.Colditz GA, Willett WC, Stampfer MJ, et al. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 8.Scuteri A, Ferrucci L. Blood pressure, arterial function, structure, and ageing: the role of hormonal replacement therapy in postmenopausal women. J Clin Hypertens (Greenwich) 2003;5:219–225. doi: 10.1111/j.1524-6175.2003.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brochu M, Tchernof A, Dionne IJ, Sites CK, Eltabbakh GH, Sims EA, et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86:1020–1025. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 10.Torrens JI, Skurnick J, Davidow AL, Korenman SG, Santoro N, Soto-Greene M, et al. Study of Women’s Health Across the Nation [SWAN]. Ethnic differences in insulin sensitivity and beta-cell function in premenopausal or early perimenopausal women without diabetes: the Study of Women’s Health Across the Nation (SWAN) Diabetes Care. 2004;27:354–361. doi: 10.2337/diacare.27.2.354. [DOI] [PubMed] [Google Scholar]
- 11.Matthews KA, Abrams B, Crawford S, Miles T, Neer R, Powell LH, et al. Body mass index in mid-life women: relative influence of menopause, hormone use, and ethnicity. Int J Obes Relat Metab Disord. 2001;25:863–873. doi: 10.1038/sj.ijo.0801618. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan GA, Kiel JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88:1973–1998. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- 13.Matthews KA, Sowers MF, Derby CA, Stein E, Miracle-McMahill H, Crawford SL, et al. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women’s Health Across the Nation (SWAN) Am Heart J. 2005;149:1066–1073. doi: 10.1016/j.ahj.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 14.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd-Jones DM, Sutton-Tyrrell K, Patel AS, Matthews KA, Pasternak RC, Everson-Rose SA, et al. Ethnic variation in hypertension among premenopausal and perimenopausal women: Study of Women’s Health Across the Nation. Hypertension. 2005;46:689–695. doi: 10.1161/01.HYP.0000182659.03194.db. [DOI] [PubMed] [Google Scholar]
- 16.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among US adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 17.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunner EJ, Marmot MG, Nanchahal K, Shipley MJ, Stansfeld SA, Juneja M, et al. Social inequality in coronary risk: central obesity and the metabolic syndrome. Evidence from the Whitehall II study. Diabetologia. 1997;40:1341–1349. doi: 10.1007/s001250050830. [DOI] [PubMed] [Google Scholar]
- 19.Gaillard TR, Schuster DP, Bossetti BM, Green PA, Osei K. The impact of socioeconomic status on cardiovascular risk factors in African-Americans at high risk for type II diabetes. Implications for syndrome X. Diabetes Care. 1997;20:745–752. doi: 10.2337/diacare.20.5.745. [DOI] [PubMed] [Google Scholar]
- 20.Winkleby MA, Kraemer HC, Ahn DK, Varady AN. Ethnic and socioeconomic differences in cardiovascular disease risk factors: findings for women from the Third National Health and Nutrition Examination Survey, 1988–1994. J Am Med Assoc. 1998;280:356–362. doi: 10.1001/jama.280.4.356. [DOI] [PubMed] [Google Scholar]
- 21.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, et al. Socioeconomic status in health research: one size does not fit all. J Am Med Assoc. 2005;294:2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 22.Shea S, Stein AD, Basch CE, Lantigua R, Maylahn C, Strogatz DS, et al. Independent associations of educational attainment and ethnicity with behavioural risk factors for cardiovascular disease. Am J Epidemiol. 1991;134:567–582. doi: 10.1093/oxfordjournals.aje.a116130. [DOI] [PubMed] [Google Scholar]
- 23.Metcalf PA, Sharrett AR, Folsom AR, Duncan BB, Patsch W, Hutchinson RG, et al. African American–white differences in lipids, lipoproteins, and apolipoproteins, by educational attainment, among middle-aged adults: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1998;148:750–760. doi: 10.1093/oxfordjournals.aje.a009696. [DOI] [PubMed] [Google Scholar]
- 24.Gallo LC, Matthews KA, Kuller LH, Sutton-Tyrrell K, Edmundowicz D. Educational attainment and coronary and aortic calcification in postmenopausal women. Psychosom Med. 2001;63:925–935. doi: 10.1097/00006842-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Lynch J, Kaplan GA, Salonen R, Cohen RD, Salonen JT. Socioeconomic status and carotid atherosclerosis. Circulation. 1995;92:1786–1792. doi: 10.1161/01.cir.92.7.1786. [DOI] [PubMed] [Google Scholar]
- 26.Lynch J, Kaplan GA, Salonen R, Salonen JT. Socioeconomic status and progression of carotid atherosclerosis. Prospective evidence from the Kuopio Ischemic Heart Disease Risk Factor Study. Arterioscler Thromb Vasc Biol. 1997;17:513–519. doi: 10.1161/01.atv.17.3.513. [DOI] [PubMed] [Google Scholar]
- 27.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors. J Am Med Assoc. 2001;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]