Abstract
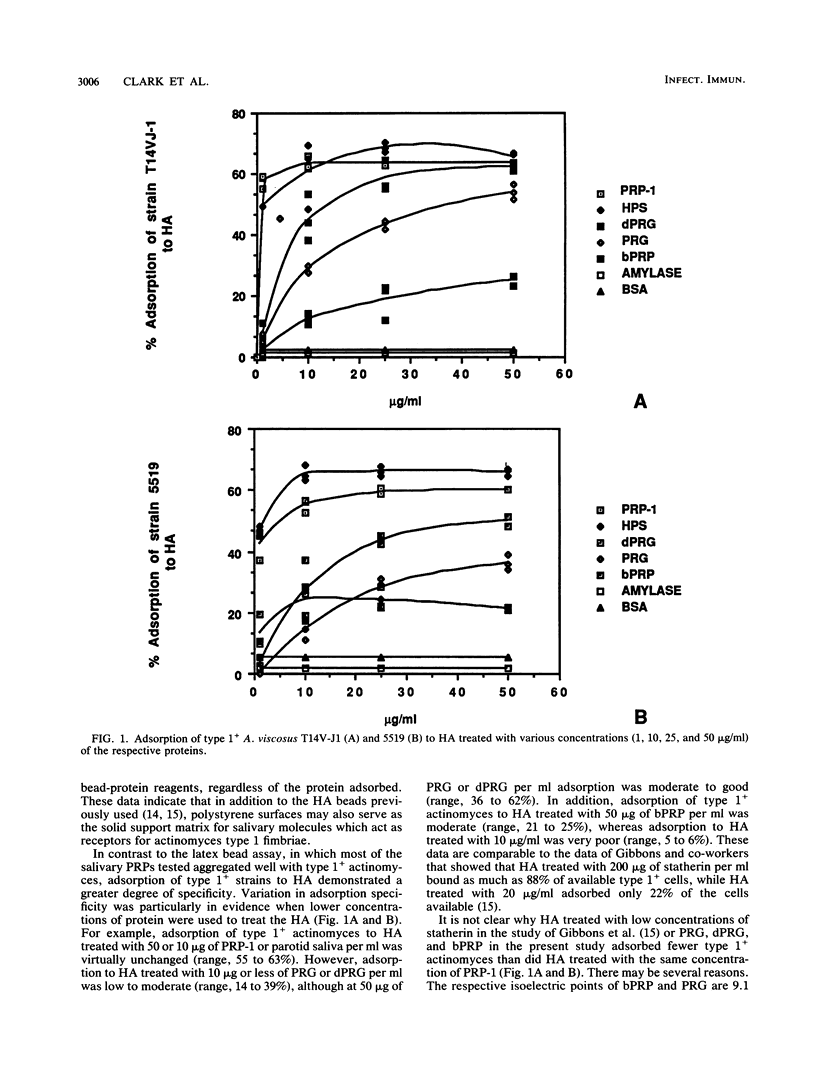
Actinomyces viscosus T14V-J1 and its fimbria-deficient mutant strain possessing type 1 fimbriae strongly aggregated with latex beads treated with acidic proline-rich protein 1, basic proline-rich proteins, and proline-rich glycoprotein and its deglycosylated derivative. These type 1+ strains did not aggregate with latex beads treated with other proteins, such as salivary amylase, salivary histidine-rich polypeptides, laminin, type 1 collagen, fibronectin, or C1q. The type 1+ strains also adsorbed well to experimental pellicles formed with acidic proline-rich protein 1, basic proline-rich proteins, and proline-rich glycoprotein and its deglycosylated derivative on hydroxyapatite (HA) surfaces. These interactions were inhibited with immunoglobulins and Fabs specific for type 1 fimbriae. Type 1- actinomyces exhibited feeble adsorption to latex beads or HA treated with any of the aforementioned proteins. Collectively, these data indicate that actinomyces type 1 fimbriae may specifically interact with several proline-rich salivary molecules, forming experimental pellicles on HA or polystyrene surfaces.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azen E., Lyons K. M., McGonigal T., Barrett N. L., Clements L. S., Maeda N., Vanin E. F., Carlson D. M., Smithies O. Clones from the human gene complex coding for salivary proline-rich proteins. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5561–5565. doi: 10.1073/pnas.81.17.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennick A., Chau G., Goodlin R., Abrams S., Tustian D., Madapallimattam G. The role of human salivary acidic proline-rich proteins in the formation of acquired dental pellicle in vivo and their fate after adsorption to the human enamel surface. Arch Oral Biol. 1983;28(1):19–27. doi: 10.1016/0003-9969(83)90022-5. [DOI] [PubMed] [Google Scholar]
- Bennick A. Salivary proline-rich proteins. Mol Cell Biochem. 1982 Jun 11;45(2):83–99. doi: 10.1007/BF00223503. [DOI] [PubMed] [Google Scholar]
- Bergey E. J., Levine M. J., Reddy M. S., Bradway S. D., Al-Hashimi I. Use of the photoaffinity cross-linking agent N-hydroxysuccinimidyl-4-azidosalicylic acid to characterize salivary-glycoprotein-bacterial interactions. Biochem J. 1986 Feb 15;234(1):43–48. doi: 10.1042/bj2340043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURBY W. A. Device for collection of human parotid saliva. J Lab Clin Med. 1953 Mar;41(3):493–496. [PubMed] [Google Scholar]
- Cisar J. O., Vatter A. E., Clark W. B., Curl S. H., Hurst-Calderone S., Sandberg A. L. Mutants of Actinomyces viscosus T14V lacking type 1, type 2, or both types of fimbriae. Infect Immun. 1988 Nov;56(11):2984–2989. doi: 10.1128/iai.56.11.2984-2989.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Wheeler T. T., Cisar J. O. Specific inhibition of adsorption of Actinomyces viscosus T14V to saliva-treated hydroxyapatite by antibody against type 1 fimbriae. Infect Immun. 1984 Feb;43(2):497–501. doi: 10.1128/iai.43.2.497-501.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Wheeler T. T., Lane M. D., Cisar J. O. Actinomyces adsorption mediated by type-1 fimbriae. J Dent Res. 1986 Sep;65(9):1166–1168. doi: 10.1177/00220345860650091001. [DOI] [PubMed] [Google Scholar]
- Crawford P. C., Clark W. B. Fimbria-specific antibodies in serum and saliva of mice immunized with Actinomyces viscosus T14V fimbriae. Infect Immun. 1986 Nov;54(2):507–515. doi: 10.1128/iai.54.2.507-515.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. E., Gebhardt B. M., Birdsell D. C. Murine model for analysis of the immune response to oral colonization. J Periodontal Res. 1981 Sep;16(5):564–573. doi: 10.1111/j.1600-0765.1981.tb02018.x. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Albumin as a blocking agent in studies of streptococcal adsorption to experimental salivary pellicles. Infect Immun. 1985 Nov;50(2):592–594. doi: 10.1128/iai.50.2.592-594.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I., Cisar J. O., Clark W. B. Adsorbed salivary proline-rich protein 1 and statherin: receptors for type 1 fimbriae of Actinomyces viscosus T14V-J1 on apatitic surfaces. Infect Immun. 1988 Nov;56(11):2990–2993. doi: 10.1128/iai.56.11.2990-2993.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I. Human salivary acidic proline-rich proteins and statherin promote the attachment of Actinomyces viscosus LY7 to apatitic surfaces. Infect Immun. 1988 Feb;56(2):439–445. doi: 10.1128/iai.56.2.439-445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. I., Bennick A., Schlesinger D. H., Minaguchi K., Madapallimattam G., Schluckebier S. K. The primary structures of six human salivary acidic proline-rich proteins (PRP-1, PRP-2, PRP-3, PRP-4, PIF-s and PIF-f). Biochem J. 1988 Oct 1;255(1):15–21. doi: 10.1042/bj2550015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. I. The isolation from human parotid saliva of a tyrosine-rich acidic peptide which exhibits high affinity for hydroxyapatite surfaces. Arch Oral Biol. 1973 Dec;18(12):1531–1541. doi: 10.1016/0003-9969(73)90128-3. [DOI] [PubMed] [Google Scholar]
- Heeb M. J., Costello A. H., Gabriel O. Characterization of a galactose-specific lectin from Actinomyces viscosus by a model aggregation system. Infect Immun. 1982 Dec;38(3):993–1002. doi: 10.1128/iai.38.3.993-1002.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman D. L., Keller P. J. The basic proline-rich proteins in human parotid saliva from a single subject. Arch Oral Biol. 1979;24(4):249–256. doi: 10.1016/0003-9969(79)90085-2. [DOI] [PubMed] [Google Scholar]
- Kauffman D., Hofmann T., Bennick A., Keller P. Basic proline-rich proteins from human parotid saliva: complete covalent structures of proteins IB-1 and IB-6. Biochemistry. 1986 May 6;25(9):2387–2392. doi: 10.1021/bi00357a013. [DOI] [PubMed] [Google Scholar]
- Kousvelari E. E., Baratz R. S., Burke B., Oppenheim F. G. Immunochemical identification and determination of proline-rich proteins in salivary secretions, enamel pellicle, and glandular tissue specimens. J Dent Res. 1980 Aug;59(8):1430–1438. doi: 10.1177/00220345800590081201. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Ellison S. A., Bahl O. P. The isolation from human parotid saliva and partial characterization of the protein core of a major parotid glycoprotein. Arch Oral Biol. 1973 Jul;18(7):827–837. doi: 10.1016/0003-9969(73)90053-8. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Weill J. C., Ellison S. A. The isolation and analysis of a glycoprotein from parotid saliva. Biochim Biophys Acta. 1969 Aug 12;188(1):165–167. doi: 10.1016/0005-2795(69)90060-9. [DOI] [PubMed] [Google Scholar]
- Li H. C., Levine M. J. Characterization of a glycopeptide from the proline-rich glycoprotein of human parotid saliva. Arch Oral Biol. 1980;25(5):353–355. doi: 10.1016/0003-9969(80)90046-1. [DOI] [PubMed] [Google Scholar]
- Loomis R. E., Bergey E. J., Levine M. J., Tabak L. A. Circular dichroism and fluorescence spectroscopic analyses of a proline-rich glycoprotein from human parotid saliva. Int J Pept Protein Res. 1985 Dec;26(6):621–629. doi: 10.1111/j.1399-3011.1985.tb03220.x. [DOI] [PubMed] [Google Scholar]
- Maeda N. Inheritance of the human salivary proline-rich proteins: a reinterpretation in terms of six loci forming two subfamilies. Biochem Genet. 1985 Jun;23(5-6):455–464. doi: 10.1007/BF00499086. [DOI] [PubMed] [Google Scholar]
- Maeda N., Kim H. S., Azen E. A., Smithies O. Differential RNA splicing and post-translational cleavages in the human salivary proline-rich protein gene system. J Biol Chem. 1985 Sep 15;260(20):11123–11130. [PubMed] [Google Scholar]
- Moreno E. C., Varughese K., Hay D. I. Effect of human salivary proteins on the precipitation kinetics of calcium phosphate. Calcif Tissue Int. 1979 Aug 24;28(1):7–16. doi: 10.1007/BF02441212. [DOI] [PubMed] [Google Scholar]
- Querinjean P., Masson P. L., Heremans J. F. Molecular weight, single-chain structure and amino acid composition of human lactoferrin. Eur J Biochem. 1971 Jun 11;20(3):420–425. doi: 10.1111/j.1432-1033.1971.tb01408.x. [DOI] [PubMed] [Google Scholar]
- Qureshi J. V., Gibbons R. J. Differences in the adsorptive behavior of human strains of Actinomyces viscosus and Actinomyces naeslundii to saliva-treated hydroxyapatite surfaces. Infect Immun. 1981 Jan;31(1):261–266. doi: 10.1128/iai.31.1.261-266.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revis G. J., Vatter A. E., Crowle A. J., Cisar J. O. Antibodies against the Ag2 fimbriae of Actinomyces viscosus T14V inhibit lactose-sensitive bacterial adherence. Infect Immun. 1982 Jun;36(3):1217–1222. doi: 10.1128/iai.36.3.1217-1222.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler T. T., Clark W. B., Birdsell D. C. Adherence of Actinomyces viscosus T14V and T14AV to hydroxyapatite surfaces in vitro and human teeth in vivo. Infect Immun. 1979 Sep;25(3):1066–1074. doi: 10.1128/iai.25.3.1066-1074.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



