Abstract
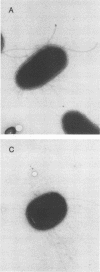
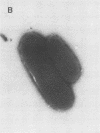
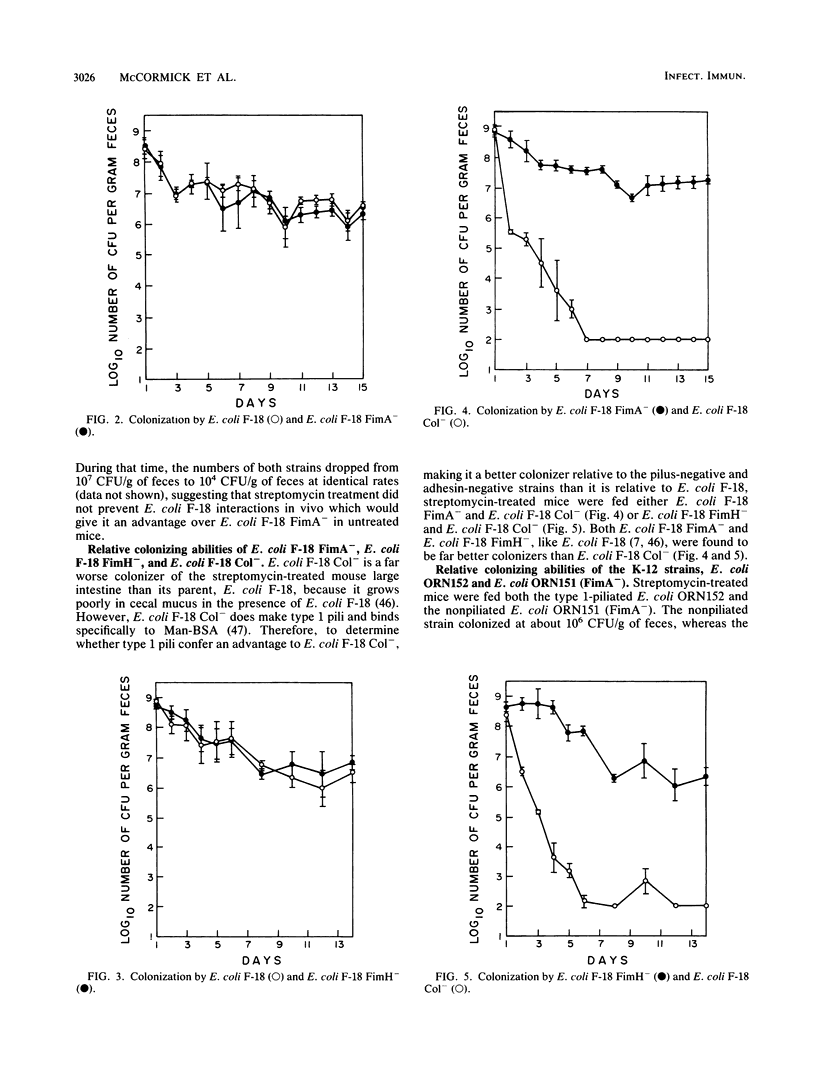
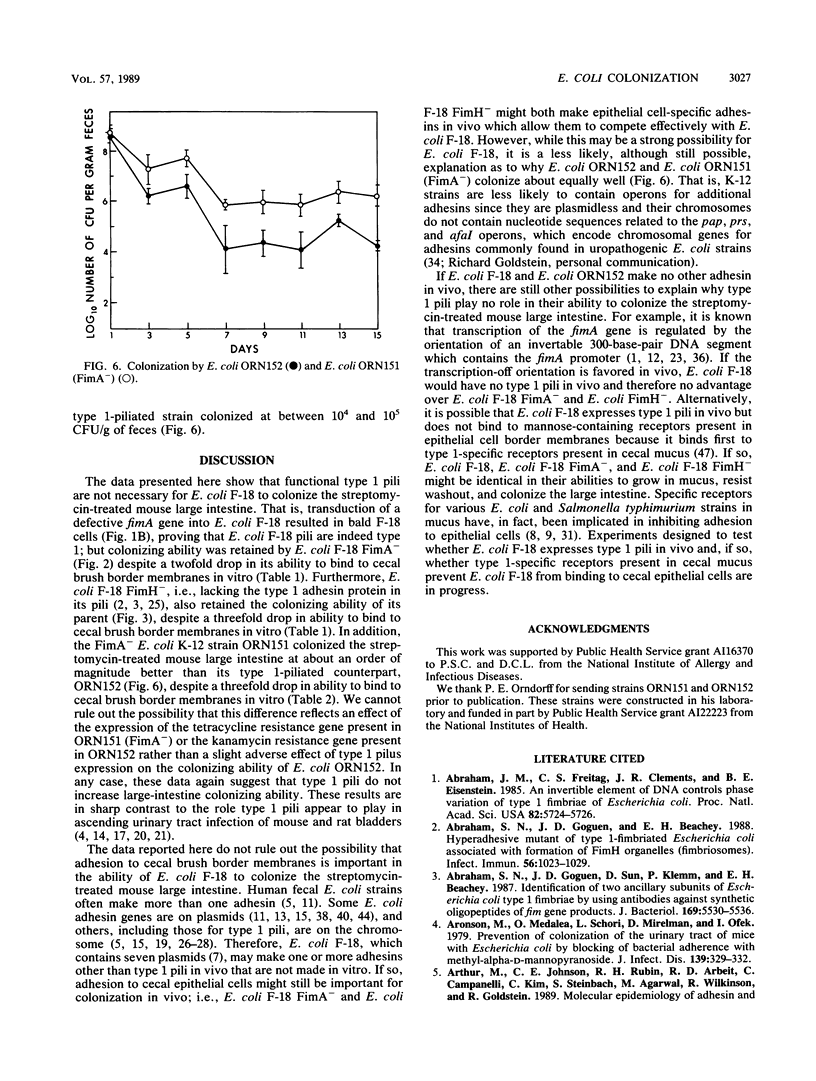
Escherichia coli F-18, an excellent colonizer of the streptomycin-treated mouse large intestine, produces type 1 pili. E. coli F-18 FimA-, type 1 pilus negative, and E. coli F-18 FimH-, type 1 pilus positive but adhesin negative, were constructed by bacteriophage P1 transduction of defective fimA and fimH genes from the E. coli K-12 strains ORN151 and ORN133, respectively, into E. coli F-18. Adhesion of E. coli F-18 to an immobilized mannose-bovine serum albumin glycoconjugate was about sixfold greater than that of either E. coli F-18 FimA- or E. coli F-18 FimH-, and adhesion of E. coli F-18 to immobilized cecal epithelial cell brush border membranes was between two- and threefold greater than that of E. coli F-18 FimA- or E. coli F-18 FimH-. When either E. coli F-18 FimA- or E. coli FimH- was fed to streptomycin-treated mice together with E. coli F-18, the pilus-negative and adhesin-negative strains colonized as well as their type 1-piliated parent. Essentially the same result was observed when the type 1-piliated E. coli K-12 strain ORN152 was fed to streptomycin-treated mice together with a nearly isogenic K-12 FimA- strain, ORN151. Furthermore, when streptomycin-treated mice were fed E. coli F-18 FimA- or E. coli F-18 FimH- together with E. coli F-18 Col-, which also makes type 1 pili but is a poor colonizer relative to E. coli F-18 because it grows poorly in mucus in the presence of E. coli F-18, the F-18 FimA- and F-18 FimH- strains colonized well (10(6) to 10(7) CFU/g of feces), whereas the number of E. coli F-18 Col- in feces decreased rapidly to 10(2) CFU/g of feces. These data show that in streptomycin-treated mice, the inability to produce functional type 1 pili has no effect on the ability of E. coli F-18 and E. coli K-12 to colonize the large intestine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. M., Freitag C. S., Clements J. R., Eisenstein B. I. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5724–5727. doi: 10.1073/pnas.82.17.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham S. N., Goguen J. D., Beachey E. H. Hyperadhesive mutant of type 1-fimbriated Escherichia coli associated with formation of FimH organelles (fimbriosomes). Infect Immun. 1988 May;56(5):1023–1029. doi: 10.1128/iai.56.5.1023-1029.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham S. N., Goguen J. D., Sun D., Klemm P., Beachey E. H. Identification of two ancillary subunits of Escherichia coli type 1 fimbriae by using antibodies against synthetic oligopeptides of fim gene products. J Bacteriol. 1987 Dec;169(12):5530–5536. doi: 10.1128/jb.169.12.5530-5536.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson M., Medalia O., Schori L., Mirelman D., Sharon N., Ofek I. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D-mannopyranoside. J Infect Dis. 1979 Mar;139(3):329–332. doi: 10.1093/infdis/139.3.329. [DOI] [PubMed] [Google Scholar]
- Cohen P. S., Arruda J. C., Williams T. J., Laux D. C. Adhesion of a human fecal Escherichia coli strain to mouse colonic mucus. Infect Immun. 1985 Apr;48(1):139–145. doi: 10.1128/iai.48.1.139-145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. S., Rossoll R., Cabelli V. J., Yang S. L., Laux D. C. Relationship between the mouse colonizing ability of a human fecal Escherichia coli strain and its ability to bind a specific mouse colonic mucous gel protein. Infect Immun. 1983 Apr;40(1):62–69. doi: 10.1128/iai.40.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean E. A., Whipp S. C., Moon H. W. Age-specific colonization of porcine intestinal epithelium by 987P-piliated enterotoxigenic Escherichia coli. Infect Immun. 1989 Jan;57(1):82–87. doi: 10.1128/iai.57.1.82-87.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm B., Roberton A. M., Sherman P. M. Inhibition of attachment of Escherichia coli RDEC-1 to intestinal microvillus membranes by rabbit ileal mucus and mucin in vitro. Infect Immun. 1988 Sep;56(9):2437–2442. doi: 10.1128/iai.56.9.2437-2442.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981 Oct 16;214(4518):337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader R. C., Davis C. P. Effect of piliation on Klebsiella pneumoniae infection in rat bladders. Infect Immun. 1980 Nov;30(2):554–561. doi: 10.1128/iai.30.2.554-561.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag C. S., Eisenstein B. I. Genetic mapping and transcriptional orientation of the fimD gene. J Bacteriol. 1983 Dec;156(3):1052–1058. doi: 10.1128/jb.156.3.1052-1058.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R. B., Bender R. A., Streicher S. L. Direct selection for P1-sensitive mutants of enteric bacteria. J Bacteriol. 1974 Jun;118(3):810–814. doi: 10.1128/jb.118.3.810-814.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahi T., Abe Y., Nakao M., Imada A., Tsuchiya K. Role of type 1 fimbriae in the pathogenesis of ascending urinary tract infection induced by escherichia coli in mice. Infect Immun. 1983 Mar;39(3):1307–1315. doi: 10.1128/iai.39.3.1307-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B. R., Maurer L., Spears P. A., Orndorff P. E. Receptor-binding function of type 1 pili effects bladder colonization by a clinical isolate of Escherichia coli. Infect Immun. 1986 Sep;53(3):693–696. doi: 10.1128/iai.53.3.693-696.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P., Christiansen G. Three fim genes required for the regulation of length and mediation of adhesion of Escherichia coli type 1 fimbriae. Mol Gen Genet. 1987 Jul;208(3):439–445. doi: 10.1007/BF00328136. [DOI] [PubMed] [Google Scholar]
- Klemm P. The fimA gene encoding the type-1 fimbrial subunit of Escherichia coli. Nucleotide sequence and primary structure of the protein. Eur J Biochem. 1984 Sep 3;143(2):395–399. doi: 10.1111/j.1432-1033.1984.tb08386.x. [DOI] [PubMed] [Google Scholar]
- Klemm P. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 1986 Jun;5(6):1389–1393. doi: 10.1002/j.1460-2075.1986.tb04372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogfelt K. A., Klemm P. Investigation of minor components of Escherichia coli type 1 fimbriae: protein chemical and immunological aspects. Microb Pathog. 1988 Mar;4(3):231–238. doi: 10.1016/0882-4010(88)90073-3. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A. F., Lark D., Schoolnik G., Falkow S. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect Immun. 1984 Oct;46(1):251–259. doi: 10.1128/iai.46.1.251-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Lindberg F., Marklund B. I., Normark S. The PapG protein is the alpha-D-galactopyranosyl-(1----4)-beta-D-galactopyranose-binding adhesin of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5898–5902. doi: 10.1073/pnas.84.16.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Marklund B. I., Strömberg N., Lindberg F., Karlsson K. A., Normark S. Uropathogenic Escherichia coli can express serologically identical pili of different receptor binding specificities. Mol Microbiol. 1988 Mar;2(2):255–263. doi: 10.1111/j.1365-2958.1988.tb00027.x. [DOI] [PubMed] [Google Scholar]
- MILLER C. P., BOHNHOFF M. CHANGES IN THE MOUSE'S ENTERIC MICROFLORA ASSOCIATED WITH ENHANCED SUSCEPTIBILITY TO SALMONELLA INFECTION FOLLOWING STREPTOMYCIN TREATMENT. J Infect Dis. 1963 Jul-Aug;113:59–66. doi: 10.1093/infdis/113.1.59. [DOI] [PubMed] [Google Scholar]
- Maurer L., Orndorff P. E. Identification and characterization of genes determining receptor binding and pilus length of Escherichia coli type 1 pili. J Bacteriol. 1987 Feb;169(2):640–645. doi: 10.1128/jb.169.2.640-645.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick B. A., Stocker B. A., Laux D. C., Cohen P. S. Roles of motility, chemotaxis, and penetration through and growth in intestinal mucus in the ability of an avirulent strain of Salmonella typhimurium to colonize the large intestine of streptomycin-treated mice. Infect Immun. 1988 Sep;56(9):2209–2217. doi: 10.1128/iai.56.9.2209-2217.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhal M. L., Laux D. C., Cohen P. S. Relative colonizing abilities of human fecal and K 12 strains of Escherichia coli in the large intestines of streptomycin-treated mice. Eur J Clin Microbiol. 1982 Jun;1(3):186–192. doi: 10.1007/BF02019621. [DOI] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S. Identification and characterization of a gene product that regulates type 1 piliation in Escherichia coli. J Bacteriol. 1984 Oct;160(1):61–66. doi: 10.1128/jb.160.1.61-66.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S. Nucleotide sequence of pilA, the gene encoding the structural component of type 1 pili in Escherichia coli. J Bacteriol. 1985 Apr;162(1):454–457. doi: 10.1128/jb.162.1.454-457.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Birch-Andersen A. Comparison of Escherichia coli fimbrial antigen F7 with type 1 fimbriae. Infect Immun. 1980 Feb;27(2):657–666. doi: 10.1128/iai.27.2.657-666.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Episome-carried surface antigen K88 of Escherichia coli. I. Transmission of the determinant of the K88 antigen and influence on the transfer of chromosomal markers. J Bacteriol. 1966 Jan;91(1):69–75. doi: 10.1128/jb.91.1.69-75.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinto M., Bender R. A. Use of bacteriophage P1 as a vector for Tn5 insertion mutagenesis. Appl Environ Microbiol. 1984 Feb;47(2):436–438. doi: 10.1128/aem.47.2.436-438.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Further observations on Escherichia coli enterotoxins with particular regard to those produced by atypical piglet strains and by calf and lamb strains: the transmissible nature of these enterotoxins and of a K antigen possessed by calf and lamb strains. J Med Microbiol. 1972 May;5(2):243–250. doi: 10.1099/00222615-5-2-243. [DOI] [PubMed] [Google Scholar]
- Wadolkowski E. A., Laux D. C., Cohen P. S. Colonization of the streptomycin-treated mouse large intestine by a human fecal Escherichia coli strain: role of adhesion to mucosal receptors. Infect Immun. 1988 May;56(5):1036–1043. doi: 10.1128/iai.56.5.1036-1043.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadolkowski E. A., Laux D. C., Cohen P. S. Colonization of the streptomycin-treated mouse large intestine by a human fecal Escherichia coli strain: role of growth in mucus. Infect Immun. 1988 May;56(5):1030–1035. doi: 10.1128/iai.56.5.1030-1035.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafriri D., Oron Y., Eisenstein B. I., Ofek I. Growth advantage and enhanced toxicity of Escherichia coli adherent to tissue culture cells due to restricted diffusion of products secreted by the cells. J Clin Invest. 1987 Apr;79(4):1210–1216. doi: 10.1172/JCI112939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waaij D., Berghuis J. M., Lekkerkerk J. E. Colonization resistance of the digestive tract of mice during systemic antibiotic treatment. J Hyg (Lond) 1972 Dec;70(4):605–610. doi: 10.1017/s0022172400022464. [DOI] [PMC free article] [PubMed] [Google Scholar]