Abstract
Pseudomonas aeruginosa is the most prominent colonizer of the respiratory tract of patients with cystic fibrosis, but it is not known why this occurs. P. aeruginosa adheres to mucins from normal individuals, but mucins from cystic fibrosis patients have not been studied. To compare adhesion to mucins from cystic fibrosis with other mucins, we prepared highly glycosylated mucin glycopeptides from cystic fibrosis and chronic bronchitis patients by ion-exchange and gel-filtration chromatography and measured the adhesion of P. aeruginosa 1244 to these glycopeptides. We found (i) that the most mucinlike glycopeptides from P. aeruginosa-infected cystic fibrosis sputa showed less bacterial adhesion than did the corresponding bronchitis samples, (ii) that the most adhesive activity in cystic fibrosis samples came from a fraction that contains O and N glycopeptides and may be in part a degradation product of P. aeruginosa infection, and (iii) that highly glycosylated glycopeptides of the most acidic species (sialylated and sulfated) showed no adhesion at all. A single cystic fibrosis sample not infected by P. aeruginosa showed better binding in the adhesion-positive fractions than did the infected sputa. These studies suggest that cystic fibrosis mucins may be altered after infection is established, resulting in less binding to some fragments. However, since the clinical picture shows heavy mucus colonization, other receptors, such as cellular glycolipids which have been shed into mucus, may be contributing to this colonization.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer E. A., Wilchek M. The use of the avidin-biotin complex as a tool in molecular biology. Methods Biochem Anal. 1980;26:1–45. doi: 10.1002/9780470110461.ch1. [DOI] [PubMed] [Google Scholar]
- Boat T. F., Cheng P. W., Wood R. E. Tracheobronchial mucus secretion in vivo and in vitro by epithelial tissues from cystic fibrosis and control subjects. Mod Probl Paediatr. 1976 Oct 24;19:141–152. [PubMed] [Google Scholar]
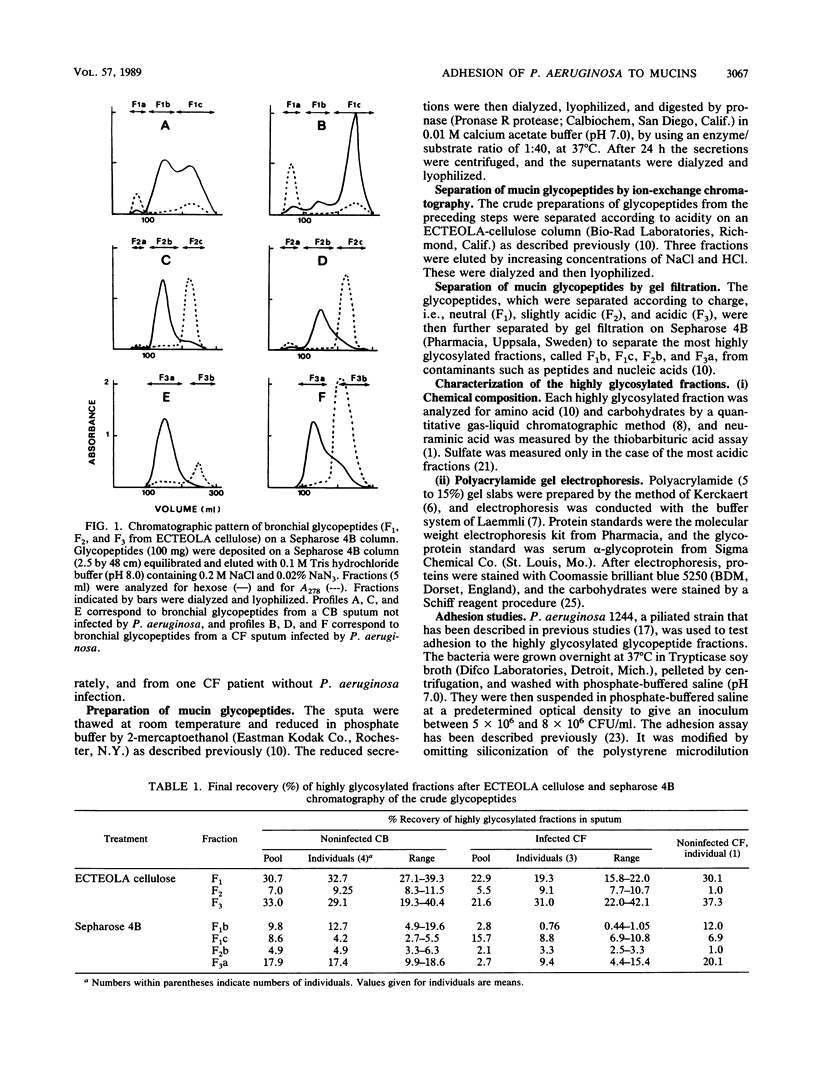
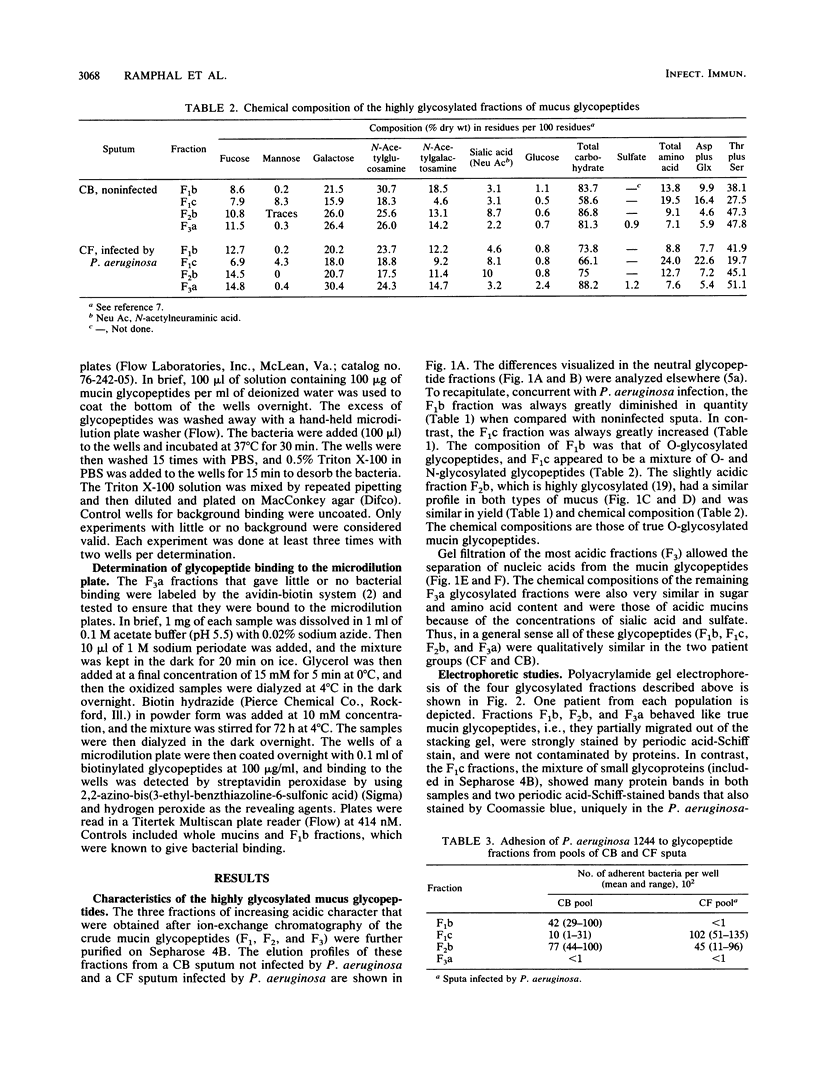
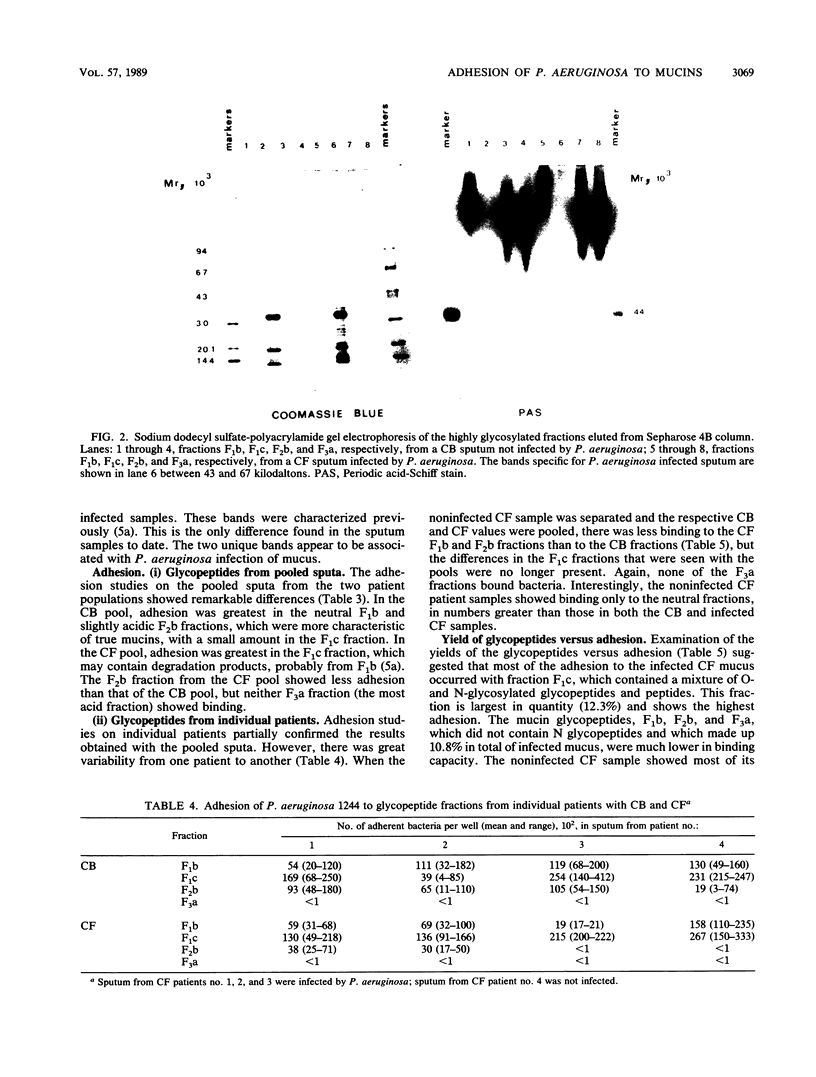
- Breg J., Van Halbeek H., Vliegenthart J. F., Lamblin G., Houvenaghel M. C., Roussel P. Structure of sialyl-oligosaccharides isolated from bronchial mucus glycoproteins of patients (blood group O) suffering from cystic fibrosis. Eur J Biochem. 1987 Oct 1;168(1):57–68. doi: 10.1111/j.1432-1033.1987.tb13387.x. [DOI] [PubMed] [Google Scholar]
- Granström M., Wretlind B., Markman B., Pavlovskis O. R., Vasil M. L. Enzyme-linked immunosorbent assay for detection of antibodies to Pseudomonas aeruginosa exoproteins. Eur J Clin Microbiol. 1985 Apr;4(2):197–200. doi: 10.1007/BF02013597. [DOI] [PubMed] [Google Scholar]
- Houdret N., Perini J. M., Galabert C., Scharfman A., Humbert P., Lamblin G., Roussel P. The high lipid content of respiratory mucins in cystic fibrosis is related to infection. Biochim Biophys Acta. 1986 Jan 15;880(1):54–61. doi: 10.1016/0304-4165(86)90119-4. [DOI] [PubMed] [Google Scholar]
- Houdret N., Ramphal R., Scharfman A., Perini J. M., Filliat M., Lamblin G., Roussel P. Evidence for the in vivo degradation of human respiratory mucins during Pseudomonas aeruginosa infection. Biochim Biophys Acta. 1989 Jul 21;992(1):96–105. doi: 10.1016/0304-4165(89)90055-x. [DOI] [PubMed] [Google Scholar]
- Kerckaert J. P. Highly simplified analytical or preparative slab gel electrophoresis. Anal Biochem. 1978 Feb;84(2):354–360. doi: 10.1016/0003-2697(78)90052-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamblin G., Boersma A., Klein A., Roussel P., van Halbeek H., Vliegenthart J. F. Primary structure determination of five sialylated oligosaccharides derived from bronchial mucus glycoproteins of patients suffering from cystic fibrosis. The occurrence of the NeuAc alpha(2----3)Gal beta(1----4)[Fuc alpha(1----3)] GlcNAc beta(1----.) structural element revealed by 500-MHz 1H NMR spectroscopy. J Biol Chem. 1984 Jul 25;259(14):9051–9058. [PubMed] [Google Scholar]
- Lamblin G., Lafitte J. J., Lhermitte M., Degand P., Roussel P. Mucins from cystic fibrosis sputum. Mod Probl Paediatr. 1976 Oct 24;19:153–164. [PubMed] [Google Scholar]
- Lamblin G., Lhermitte M., Degand P., Roussel P., Slayter H. S. Chemical and physical properties of human bronchial mucus glycoproteins. Biochimie. 1979;61(1):23–43. doi: 10.1016/s0300-9084(79)80310-7. [DOI] [PubMed] [Google Scholar]
- Leprat R., Michel-Briand Y. Extracellular neuraminidase production by a strain of Pseudomonas aeruginosa isolated from cystic fibrosis. Ann Microbiol (Paris) 1980 Nov-Dec;131B(3):209–222. [PubMed] [Google Scholar]
- Mawhinney T. P., Adelstein E., Morris D. A., Mawhinney A. M., Barbero G. J. Structure determination of five sulfated oligosaccharides derived from tracheobronchial mucus glycoproteins. J Biol Chem. 1987 Mar 5;262(7):2994–3001. [PubMed] [Google Scholar]
- Parsons C. L., Mulholland S. G., Anwar H. Antibacterial activity of bladder surface mucin duplicated by exogenous glycosaminoglycan (heparin). Infect Immun. 1979 May;24(2):552–557. doi: 10.1128/iai.24.2.552-557.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncz L., Jentoft N., Ho M. C., Dearborn D. G. Kinetics of proteolysis of hog gastric mucin by human neutrophil elastase and by Pseudomonas aeruginosa elastase. Infect Immun. 1988 Mar;56(3):703–704. doi: 10.1128/iai.56.3.703-704.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Guay C., Pier G. B. Pseudomonas aeruginosa adhesins for tracheobronchial mucin. Infect Immun. 1987 Mar;55(3):600–603. doi: 10.1128/iai.55.3.600-603.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Sadoff J. C., Pyle M., Silipigni J. D. Role of pili in the adherence of Pseudomonas aeruginosa to injured tracheal epithelium. Infect Immun. 1984 Apr;44(1):38–40. doi: 10.1128/iai.44.1.38-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. C., Brown C. F., Jacoby J. Z., 3rd, Lynn W. S., Kaufman B. Biochemical properties of tracheobronchial mucins from cystic fibrosis and non-cystic fibrosis individuals. Pediatr Res. 1987 Nov;22(5):545–551. doi: 10.1203/00006450-198711000-00015. [DOI] [PubMed] [Google Scholar]
- Roussel P., Lamblin G., Degand P. Heterogeneity of the carbohydrate chains of sulfated bronchial glycoproteins isolated from a patient suffering from cystic fibrosis. J Biol Chem. 1975 Mar 25;250(6):2114–2122. [PubMed] [Google Scholar]
- SPENCER B. The ultramicro determination of inorganic sulphte. Biochem J. 1960 Jun;75:435–440. doi: 10.1042/bj0750435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayter H. S., Lamblin G., Le Treut A., Galabert C., Houdret N., Degand P., Roussel P. Complex structure of human bronchial mucus glycoprotein. Eur J Biochem. 1984 Jul 16;142(2):209–218. doi: 10.1111/j.1432-1033.1984.tb08273.x. [DOI] [PubMed] [Google Scholar]
- Tang P. W., Scudder P., Mehmet H., Hounsell E. F., Feizi T. Sulphate groups are involved in the antigenicity of keratan sulphate and mask i antigen expression on their poly-N-acetyllactosamine backbones. An immunochemical and chromatographic study of keratan sulphate oligosaccharides after desulphation or nitrosation. Eur J Biochem. 1986 Nov 3;160(3):537–545. doi: 10.1111/j.1432-1033.1986.tb10072.x. [DOI] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Adherence of Pseudomonas aeruginosa to human tracheobronchial mucin. Infect Immun. 1984 Jul;45(1):197–202. doi: 10.1128/iai.45.1.197-202.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Tracheobronchial mucin receptor for Pseudomonas aeruginosa: predominance of amino sugars in binding sites. Infect Immun. 1985 May;48(2):331–335. doi: 10.1128/iai.48.2.331-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]