Abstract
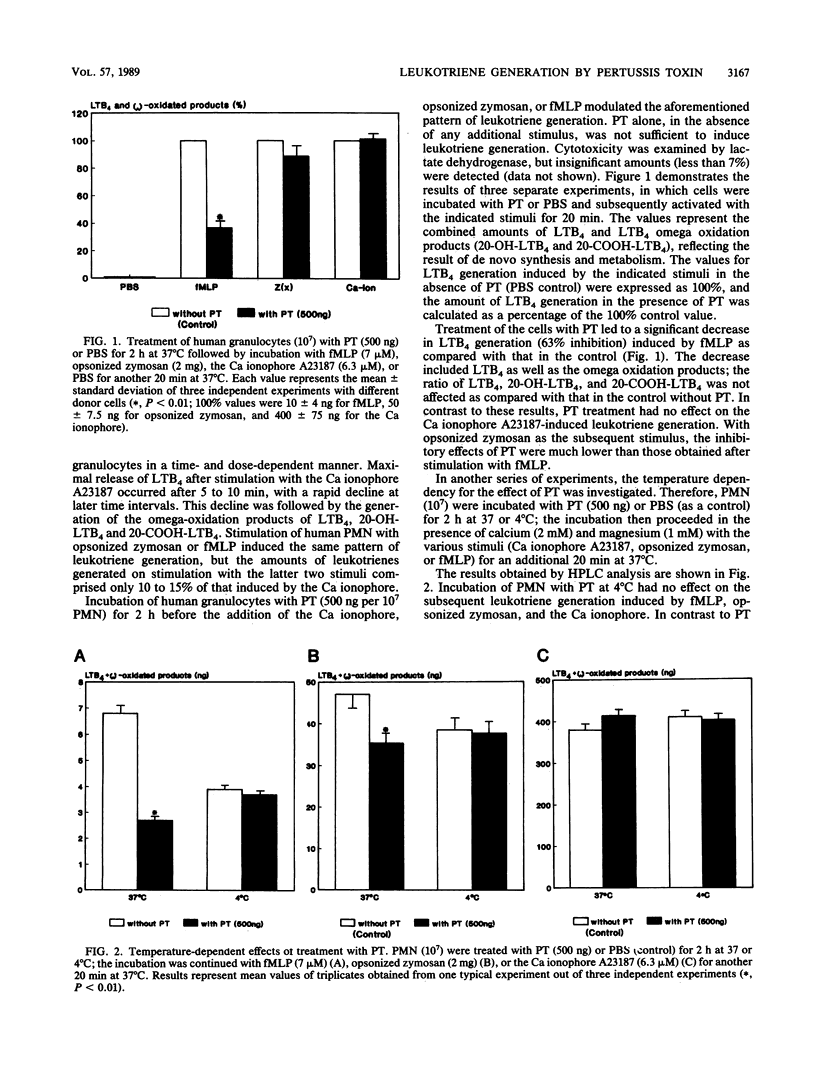
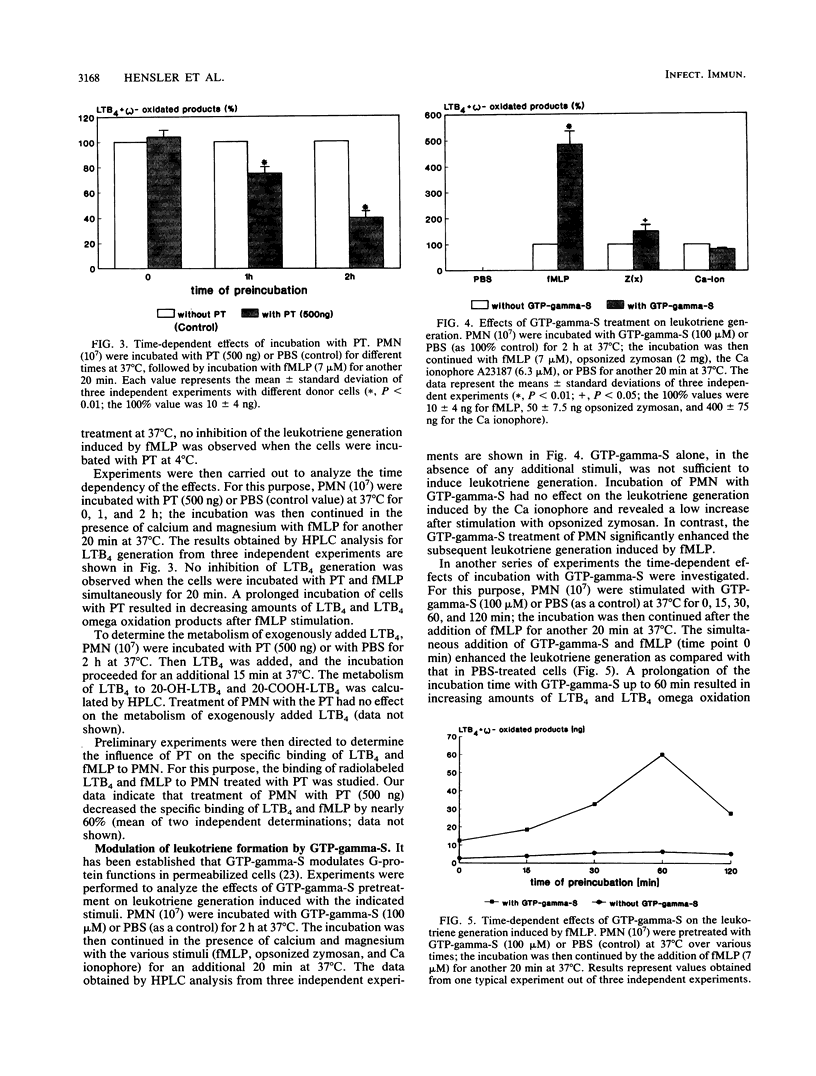
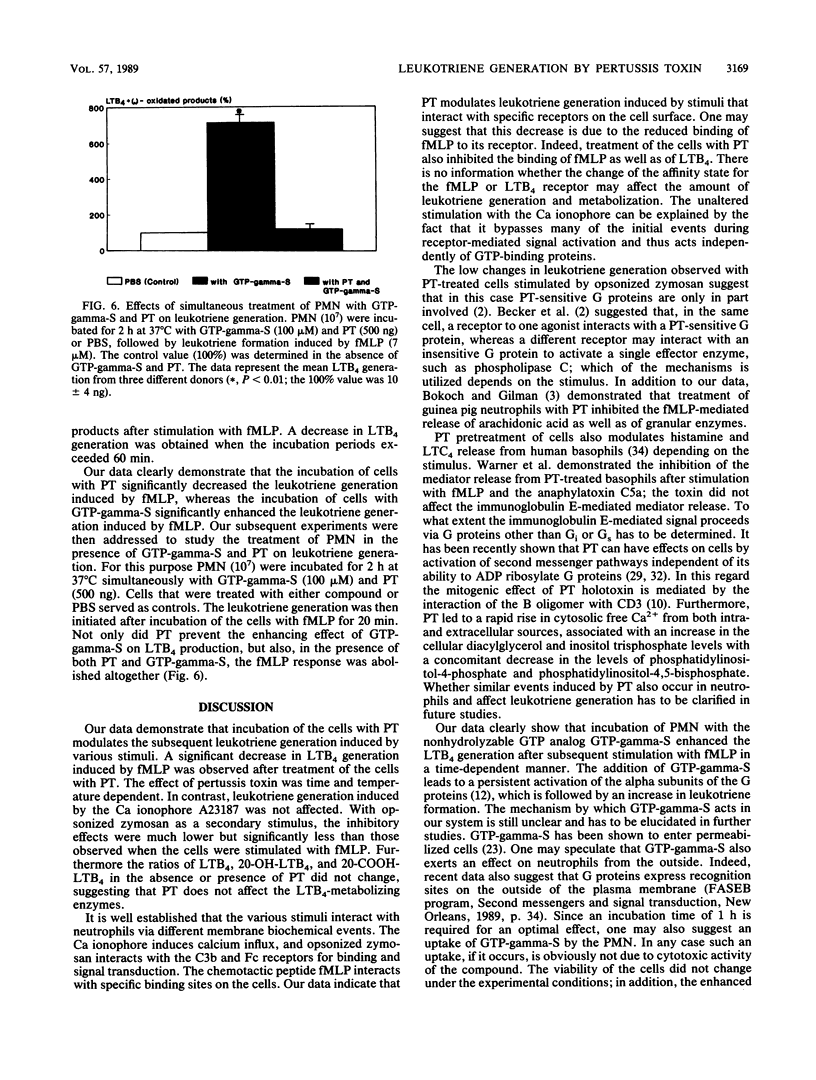
The purpose of our study was to characterize the properties of the interaction of pertussis toxin with human polymorphonuclear leukocytes for the modulation of leukotriene generation and metabolism. The cells were stimulated with either the Ca ionophore A23187, opsonized zymosan, or the bacterial peptide formyl-methionyl-leucyl phenylalanine. Incubation of the cells with pertussis toxin led to a rapid inhibition of LTB4 generation when formyl-methionyl-leucyl phenylalanine was used as the stimulus, whereas there was no effect with the Ca ionophore and just a low effect with opsonized zymosan. The inhibition of leukotriene generation was dependent on the incubation time, temperature, and pertussis toxin concentration. The effect was not dependent on the presence of calcium. Incubation of the cells with guanosine 5'-O-(3-thiotriphosphate) the stable analog of GTP, led to a time-dependent increase in leukotriene generation induced by formyl-methionyl-leucyl phenylalanine which was abolished by the simultaneous addition of pertussis toxin. Our data suggest that the formyl-methionyl-leucyl phenylalanine-induced generation of leukotrienes is dependent on a GTP-binding protein. The participation of the various G proteins has yet to be elucidated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswanikumar S., Corcoran B., Schiffmann E., Day A. R., Freer R. J., Showell H. J., Becker E. L. Demonstration of a receptor on rabbit neutrophils for chemotactic peptides. Biochem Biophys Res Commun. 1977 Jan 24;74(2):810–817. doi: 10.1016/0006-291x(77)90375-8. [DOI] [PubMed] [Google Scholar]
- Becker E. L., Kanaho Y., Kermode J. C. Nature and functioning of the pertussis toxin-sensitive G protein of neutrophils. Biomed Pharmacother. 1987;41(6):289–297. [PubMed] [Google Scholar]
- Bokoch G. M., Gilman A. G. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984 Dec;39(2 Pt 1):301–308. doi: 10.1016/0092-8674(84)90008-4. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Reed P. W. Inhibition of the neutrophil oxidative response to a chemotactic peptide by inhibitors of arachidonic acid oxygenation. Biochem Biophys Res Commun. 1979 Sep 27;90(2):481–487. doi: 10.1016/0006-291x(79)91260-9. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Reed P. W. Stimulation of arachidonic acid metabolism in the polymorphonuclear leukocyte by an N-formylated peptide. Comparison with ionophore A23187. J Biol Chem. 1980 Nov 10;255(21):10223–10226. [PubMed] [Google Scholar]
- Bray M. A. Leukotrienes in inflammation. Agents Actions. 1986 Oct;19(1-2):87–99. doi: 10.1007/BF01977263. [DOI] [PubMed] [Google Scholar]
- Brom J., Schönfeld W., König W. Metabolism of leukotriene B4 by activated human polymorphonuclear granulocytes. Immunology. 1988 Jul;64(3):509–518. [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gray L. S., Huber K. S., Gray M. C., Hewlett E. L., Engelhard V. H. Pertussis toxin effects on T lymphocytes are mediated through CD3 and not by pertussis toxin catalyzed modification of a G protein. J Immunol. 1989 Mar 1;142(5):1631–1638. [PubMed] [Google Scholar]
- Hansson G., Lindgren J. A., Dahlén S. E., Hedqvist P., Samuelsson B. Identification and biological activity of novel omega-oxidized metabolites of leukotriene B4 from human leukocytes. FEBS Lett. 1981 Jul 20;130(1):107–112. doi: 10.1016/0014-5793(81)80676-x. [DOI] [PubMed] [Google Scholar]
- Harnett M. M., Klaus G. G. G protein regulation of receptor signalling. Immunol Today. 1988 Oct;9(10):315–320. doi: 10.1016/0167-5699(88)91325-4. [DOI] [PubMed] [Google Scholar]
- Jubiz W., Rådmark O., Malmsten C., Hansson G., Lindgren J. A., Palmblad J., Udén A. M., Samuelsson B. A novel leukotriene produced by stimulation of leukocytes with formylmethionylleucylphenylalanine. J Biol Chem. 1982 Jun 10;257(11):6106–6110. [PubMed] [Google Scholar]
- Katada T., Ui M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J Biol Chem. 1982 Jun 25;257(12):7210–7216. [PubMed] [Google Scholar]
- Knöller J., Schönfeld W., Köller M., Hensler T., König W. Arachidonic acid metabolites from polymorphonuclear leukocytes of healthy donors, severely burned patients and children with cystic fibrosis--routine monitoring by high-performance liquid chromatography. J Chromatogr. 1988 Jun 3;427(2):199–208. doi: 10.1016/0378-4347(88)80122-1. [DOI] [PubMed] [Google Scholar]
- Koo C., Lefkowitz R. J., Snyderman R. Guanine nucleotides modulate the binding affinity of the oligopeptide chemoattractant receptor on human polymorphonuclear leukocytes. J Clin Invest. 1983 Sep;72(3):748–753. doi: 10.1172/JCI111045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köller M., Schönfeld W., Knöller J., Bremm K. D., König W., Spur B., Crea A., Peters W. The metabolism of leukotrienes in blood plasma studied by high-performance liquid chromatography. Biochim Biophys Acta. 1985 Jan 9;833(1):128–134. doi: 10.1016/0005-2760(85)90260-7. [DOI] [PubMed] [Google Scholar]
- König W., Frickhofen N., Tesch H. Generation and secretion of eosinophilotactic activity from human polymorphonuclear neutrophils by various mechanisms of cell activation. Immunology. 1979 Apr;36(4):733–742. [PMC free article] [PubMed] [Google Scholar]
- König W., Kroegel C., Kunau H. W., Borgeat P. Comparison of the eosinophil chemotactic factor (ECF) with endogeneous hydroxyeicosatetraenoic acids of leukocytes. Int Arch Allergy Appl Immunol. 1981;66 (Suppl 1):168–171. doi: 10.1159/000232895. [DOI] [PubMed] [Google Scholar]
- Lanni C., Becker E. L. Release of phospholipase A2 activity from rabbit peritoneal neutrophils by f-Met-Leu-Phe. Am J Pathol. 1983 Oct;113(1):90–94. [PMC free article] [PubMed] [Google Scholar]
- Lindgren J. A., Hansson G., Samuelsson B. Formation of novel hydroxylated eicosatetraenoic acids in preparations of human polymorphonuclear leukocytes. FEBS Lett. 1981 Jun 15;128(2):329–335. doi: 10.1016/0014-5793(81)80110-x. [DOI] [PubMed] [Google Scholar]
- Nakashima S., Nagata K., Ueeda K., Nozawa Y. Stimulation of arachidonic acid release by guanine nucleotide in saponin-permeabilized neutrophils: evidence for involvement of GTP-binding protein in phospholipase A2 activation. Arch Biochem Biophys. 1988 Mar;261(2):375–383. doi: 10.1016/0003-9861(88)90353-0. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J., Kosfeld S., Nishihira J. Binding and metabolism of leukotriene B4 by neutrophils and their subcellular organelles. J Cell Physiol. 1986 Mar;126(3):359–370. doi: 10.1002/jcp.1041260306. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Salmon J. A. Release of leukotriene B4 from human neutrophils and its relationship to degranulation induced by N-formyl-methionyl-leucyl-phenylalanine, serum-treated zymosan and the ionophore A23187. Immunology. 1983 Sep;50(1):65–73. [PMC free article] [PubMed] [Google Scholar]
- Patrone F., Dallegri F., Sacchetti C. Stimulation of granulocyte adhesiveness by the chemotactic peptide N-formyl-L-methionyl-L-phenylalanine. Res Exp Med (Berl) 1980;177(1):19–22. doi: 10.1007/BF01851628. [DOI] [PubMed] [Google Scholar]
- Raulf M., König W. Modulation of leukotriene release from human polymorphonuclear leucocytes by PMA and arachidonic acid. Immunology. 1988 May;64(1):51–59. [PMC free article] [PubMed] [Google Scholar]
- Raulf M., Stüning M., König W. Effect of cations on leukotriene release: requirements for the metabolism of peptido-leukotrienes (leukotrienes C4, D4) by human polymorphonuclear granulocytes. Immunology. 1986 Jul;58(3):479–487. [PMC free article] [PubMed] [Google Scholar]
- Rosoff P. M., Walker R., Winberry L. Pertussis toxin triggers rapid second messenger production in human T lymphocytes. J Immunol. 1987 Oct 1;139(7):2419–2423. [PubMed] [Google Scholar]
- Snyderman R., Goetzl E. J. Molecular and cellular mechanisms of leukocyte chemotaxis. Science. 1981 Aug 21;213(4510):830–837. doi: 10.1126/science.6266014. [DOI] [PubMed] [Google Scholar]
- Thom R. E., Casnellie J. E. Pertussis toxin activates protein kinase C and a tyrosine protein kinase in the human T cell line Jurkat. FEBS Lett. 1989 Feb 13;244(1):181–184. doi: 10.1016/0014-5793(89)81188-3. [DOI] [PubMed] [Google Scholar]
- Warner J. A., Yancey K. B., MacGlashan D. W., Jr The effect of pertussis toxin on mediator release from human basophils. J Immunol. 1987 Jul 1;139(1):161–165. [PubMed] [Google Scholar]


