Abstract
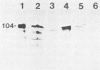
A homologous polyclonal antibody was produced in a rabbit to the 104-kilodalton (kDa) protein hemolysin of Actinobacillus pleuropneumoniae serotype 1 strain CM-5. In immunoblots, this antibody recognized a similar 104-kDa protein produced in culture supernatants by A. pleuropneumoniae serotypes 1 to 12 and taxon "Minor group" in addition to Pasteurella haemolytica, Actinobacillus suis, and alpha-hemolysin-producing Escherichia coli (but only weakly in the latter two organisms). These results were reproduced by using a mouse monoclonal antibody to the CM-5 104-kDa protein hemolysin, except that the monoclonal antibody bound more strongly to the alpha-hemolysin produced by E. coli, only weakly to the 104-kDa protein produced by "Minor group," and not at all to any extracellular antigens produced by A. suis. Pigs experimentally infected with A. pleuropneumoniae serotypes 1 to 10 and A. suis produced an antibody that recognized the 104-kDa hemolysin produced by CM-5. A pig challenged with a "Minor group" strain did not have such antibodies. Rabbit antiserum produced against the leukotoxin of P. haemolytica and alpha-hemolysin-producing E. coli also recognized the CM-5 hemolysin, but the latter only weakly. The hemolytic activity produced by CM-5 in culture supernatant was neutralized strongly by the pig serum to serotypes 1, 2, 5, 6, 9, and 10 and A. suis, only partially by serotype 8 antiserum and the rabbit antiserum to P. haemolytica leukotoxin, and not all by the antiserum to serotypes 3, 4, and 7 and "Minor group" and the E. coli alpha-hemolysin. These results indicate that a similar but not identical 104-kDa protein is produced in vitro and in vivo by all serotypes of A. pleuropneumoniae and may be related to cytolysins produced by other gram-negative bacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Schmid A., Wagner W., Goebel W. Pore formation by the Escherichia coli hemolysin: evidence for an association-dissociation equilibrium of the pore-forming aggregates. Infect Immun. 1989 Mar;57(3):887–895. doi: 10.1128/iai.57.3.887-895.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenish J., Rosendal S. Identification of the heat-labile hemolysin of Actinobacillus pleuropneumoniae serotype 1. Can J Vet Res. 1989 Apr;53(2):251–254. [PMC free article] [PubMed] [Google Scholar]
- Eberspächer B., Hugo F., Bhakdi S. Quantitative study of the binding and hemolytic efficiency of Escherichia coli hemolysin. Infect Immun. 1989 Mar;57(3):983–988. doi: 10.1128/iai.57.3.983-988.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., Nicolet J. Regulation of hemolysin expression in Actinobacillus pleuropneumoniae serotype 1 by Ca2+. Infect Immun. 1988 Oct;56(10):2570–2575. doi: 10.1128/iai.56.10.2570-2575.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen A. E., Bertram T. A. Morphological and biochemical comparison of virulent and avirulent isolates of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1986 Feb;51(2):419–424. doi: 10.1128/iai.51.2.419-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggett A. D., Harrison L. R., Farrell R. L. Acute inflammatory effects of intratracheally instilled Escherichia coli endotoxin and sonicated suspension of Haemophilus pleuropneumoniae in swine. Can J Vet Res. 1986 Oct;50(4):526–531. [PMC free article] [PubMed] [Google Scholar]
- Lo R. Y., Shewen P. E., Strathdee C. A., Greer C. N. Cloning and expression of the leukotoxin gene of Pasteurella haemolytica A1 in Escherichia coli K-12. Infect Immun. 1985 Dec;50(3):667–671. doi: 10.1128/iai.50.3.667-671.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley J. R., Kadis S., Mayberry W. R. Isolation, purification, and partial characterization of a lipopolysaccharide from Haemophilus pleuropneumoniae. Infect Immun. 1986 Feb;51(2):501–506. doi: 10.1128/iai.51.2.501-506.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R. Haemophilus pleuropneumoniae serotypes--cross protection experiments. Nord Vet Med. 1984 Jul-Aug;36(7-8):221–234. [PubMed] [Google Scholar]
- Rosendal S., Boyd D. A., Gilbride K. A. Comparative virulence of porcine Haemophilus bacteria. Can J Comp Med. 1985 Jan;49(1):68–74. [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Devenish J., MacInnes J. I., Lumsden J. H., Watson S., Xun H. Evaluation of heat-sensitive, neutrophil-toxic, and hemolytic activity of Haemophilus (Actinobacillus) pleuropneumoniae. Am J Vet Res. 1988 Jul;49(7):1053–1058. [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Udeze F. A., Latimer K. S., Kadis S. Role of haemophilus pleuropneumoniae lipopolysaccharide endotoxin in the pathogenesis of porcine Haemophilus pleuropneumonia. Am J Vet Res. 1987 May;48(5):768–773. [PubMed] [Google Scholar]