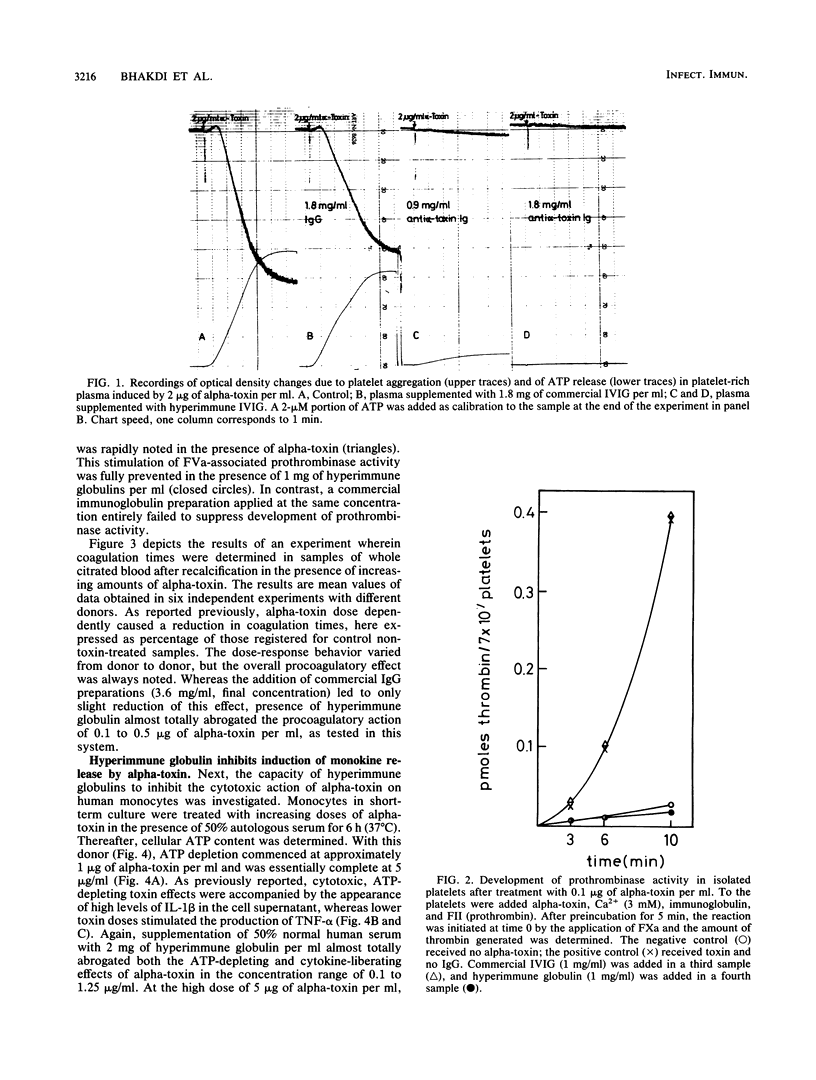
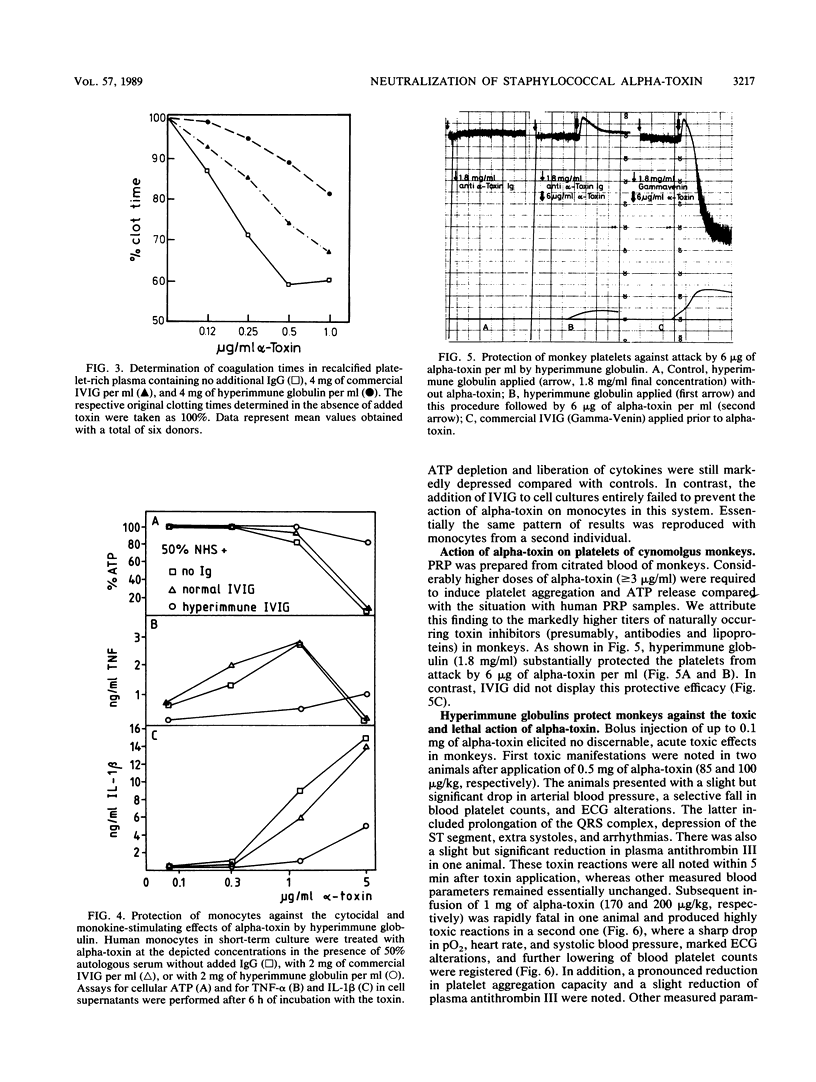
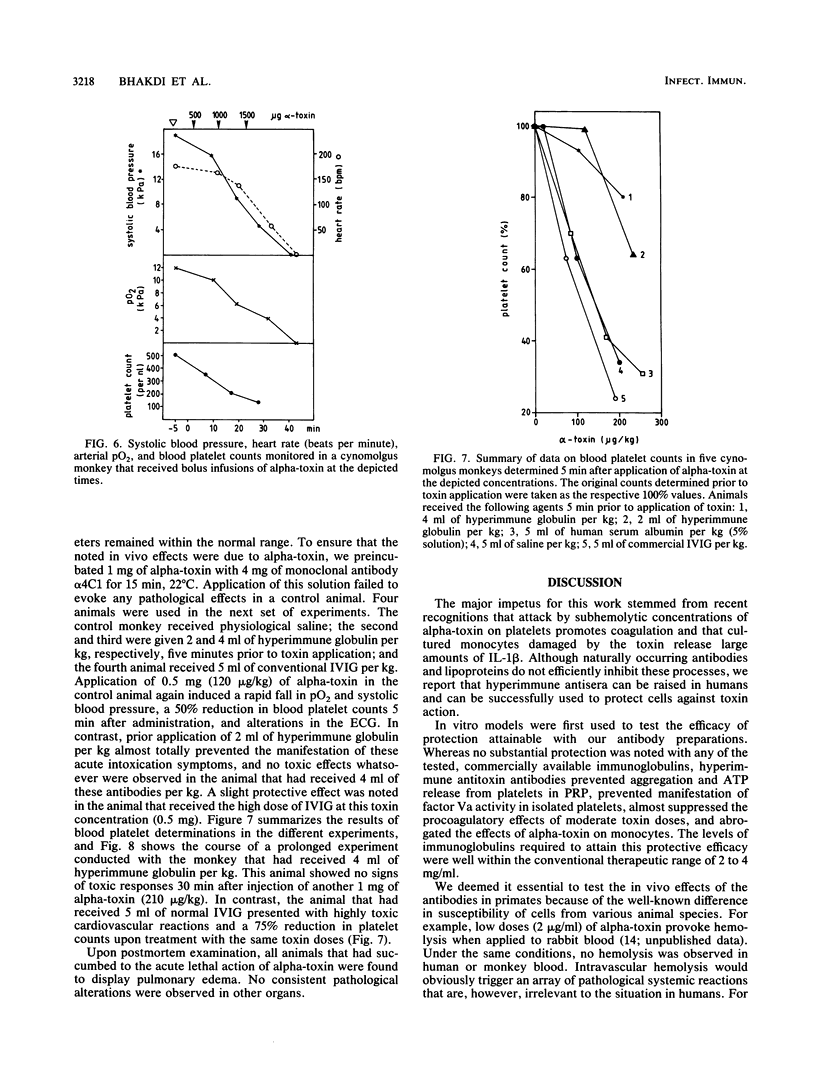
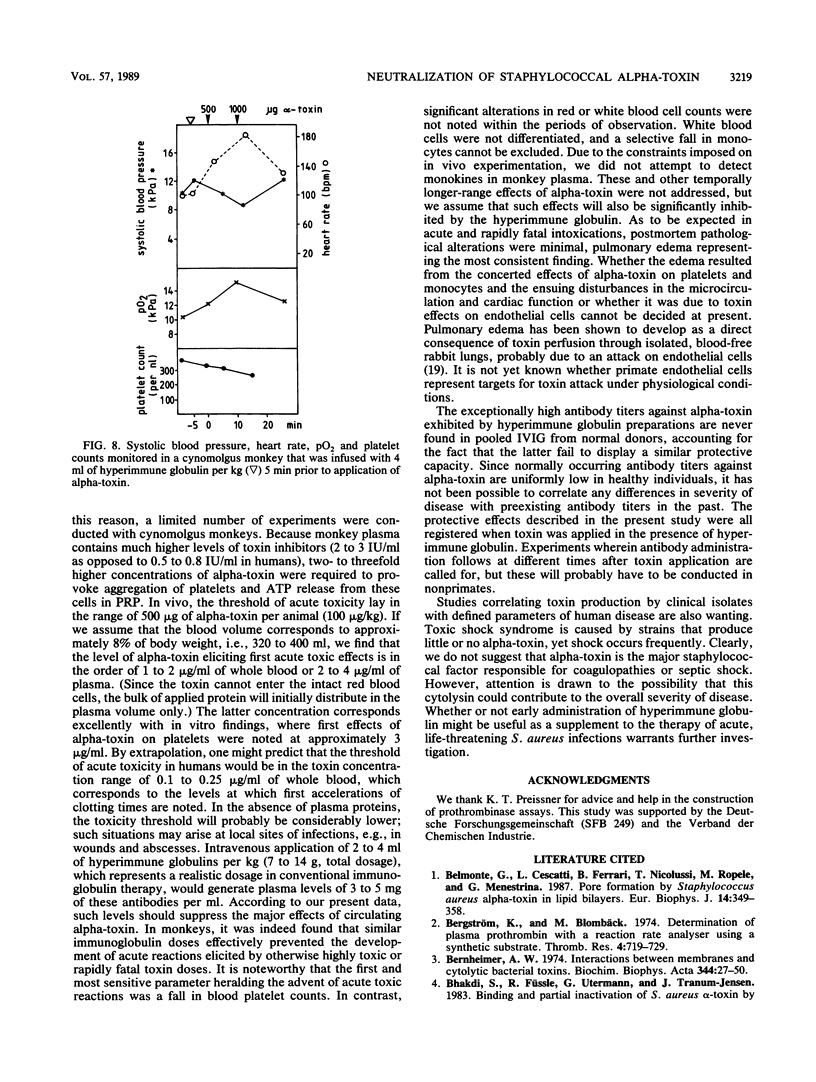
Abstract
Alpha-toxin, the major cytolysin of Staphylococcus aureus, preferentially attacks human platelets and cultured monocytes, thereby promoting coagulation and the release of interleukin-1 and tumor necrosis factor. Titers of naturally occurring antibodies in human blood are not high enough to substantially inhibit these pathological reactions. In the present study, F(ab')2 fragment preparations from hyperimmune globulin obtained from immunized volunteers were tested for their capacity to inhibit the cytotoxic action of alpha-toxin in vitro and in vivo. These antibody preparations exhibited neutralizing anti-alpha-toxin titers of 80 to 120 IU/ml, whereas titers in commercial immunoglobulin preparations were 1 to 4 IU/ml. In vitro, the presence of 2 to 4 mg of hyperimmune globulin per ml protected human platelets against the action of 1 to 2 micrograms of alpha-toxin per ml. Similarly, these antibodies fully protected human monocytes against the ATP-depleting and cytokine-liberating effects of 0.1 to 1 microgram of alpha-toxin per ml. Intravenous application of 0.5 mg (85 to 120 micrograms/kg of body weight) of alpha-toxin in cynomolgus monkeys elicited acute pathophysiological reactions which were heralded by a selective drop in blood platelet counts. Toxin doses of 1 to 2 mg (170 to 425 micrograms/kg) had a rapid lethal effect, the animals presenting with signs of cardiovascular collapse and pulmonary edema. Prior intravenous application of 4 ml of hyperimmune globulins per kg inhibited the systemic toxic and lethal effects of 1 mg (200 micrograms/kg) of alpha-toxin. In contrast, normal human immunoglobulins exhibited no substantial protective efficacy in vitro and only marginal effects in vivo. It is concluded that high-titered anti-alpha-toxin antibodies effectively protect against the cytotoxic actions of alpha-toxin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belmonte G., Cescatti L., Ferrari B., Nicolussi T., Ropele M., Menestrina G. Pore formation by Staphylococcus aureus alpha-toxin in lipid bilayers. Dependence upon temperature and toxin concentration. Eur Biophys J. 1987;14(6):349–358. doi: 10.1007/BF00262320. [DOI] [PubMed] [Google Scholar]
- Bergström K., Blombäck M. Determination of plasma prothrombin with a reaction rate analyzer using a synthetic substrate. Thromb Res. 1974 Jun;4(6):719–729. doi: 10.1016/0049-3848(74)90016-4. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Füssle R. Correlation between toxin binding and hemolytic activity in membrane damage by staphylococcal alpha-toxin. Infect Immun. 1984 Nov;46(2):318–323. doi: 10.1128/iai.46.2.318-323.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Mannhardt U., Hugo F., Klapettek K., Mueller-Eckhardt C., Roka L. Staphylococcal alpha toxin promotes blood coagulation via attack on human platelets. J Exp Med. 1988 Aug 1;168(2):527–542. doi: 10.1084/jem.168.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to mammalian cells by proteins that form transmembrane pores. Rev Physiol Biochem Pharmacol. 1987;107:147–223. doi: 10.1007/BFb0027646. [DOI] [PubMed] [Google Scholar]
- Dahlbäck B., Stenflo J. The activation of prothrombin by platelet-bound factor Xa. Eur J Biochem. 1980 Mar;104(2):549–557. doi: 10.1111/j.1432-1033.1980.tb04458.x. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Fasco M. J. Polyethylene glycol 6,000 enhancement of the clotting of fibrinogen solutions in visual and mechanical assays. Thromb Res. 1974 Jun;4(6):809–817. doi: 10.1016/0049-3848(74)90024-3. [DOI] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. S., Kehoe M. Primary sequence of the alpha-toxin gene from Staphylococcus aureus wood 46. Infect Immun. 1984 Nov;46(2):615–618. doi: 10.1128/iai.46.2.615-618.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman S. Action of staphylococcal alpha-toxin on membranes: some recent advances. Mol Cell Biochem. 1979 Feb 9;23(3):143–152. doi: 10.1007/BF00219453. [DOI] [PubMed] [Google Scholar]
- Hugo F., Sinner A., Reichwein J., Bhakdi S. Quantitation of monomeric and oligomeric forms of membrane-bound staphylococcal alpha-toxin by enzyme-linked immunosorbent assay with a neutralizing monoclonal antibody. Infect Immun. 1987 Dec;55(12):2933–2939. doi: 10.1128/iai.55.12.2933-2939.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menestrina G. Ionic channels formed by Staphylococcus aureus alpha-toxin: voltage-dependent inhibition by divalent and trivalent cations. J Membr Biol. 1986;90(2):177–190. doi: 10.1007/BF01869935. [DOI] [PubMed] [Google Scholar]
- O'Reilly M., de Azavedo J. C., Kennedy S., Foster T. J. Inactivation of the alpha-haemolysin gene of Staphylococcus aureus 8325-4 by site-directed mutagenesis and studies on the expression of its haemolysins. Microb Pathog. 1986 Apr;1(2):125–138. doi: 10.1016/0882-4010(86)90015-x. [DOI] [PubMed] [Google Scholar]
- Preissner K. T., Wassmuth R., Müller-Berghaus G. Physicochemical characterization of human S-protein and its function in the blood coagulation system. Biochem J. 1985 Oct 15;231(2):349–355. doi: 10.1042/bj2310349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogolsky M. Nonenteric toxins of Staphylococcus aureus. Microbiol Rev. 1979 Sep;43(3):320–360. doi: 10.1128/mr.43.3.320-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger W., Bauer M., Bhakdi S. Staphylococcal alpha-toxin elicits hypertension in isolated rabbit lungs. Evidence for thromboxane formation and the role of extracellular calcium. J Clin Invest. 1984 Sep;74(3):849–858. doi: 10.1172/JCI111502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Sensitive assay for detection of toxin-induced damage to the cytoplasmic membrane of human diploid fibroblasts. Infect Immun. 1975 Aug;12(2):225–232. doi: 10.1128/iai.12.2.225-232.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobkes N., Wallace B. A., Bayley H. Secondary structure and assembly mechanism of an oligomeric channel protein. Biochemistry. 1985 Apr 9;24(8):1915–1920. doi: 10.1021/bi00329a017. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Tomita T., Yasuda T. Membrane-damaging action of staphylococcal alpha-toxin on phospholipid-cholesterol liposomes. Biochim Biophys Acta. 1987 Apr 23;898(3):257–265. doi: 10.1016/0005-2736(87)90065-4. [DOI] [PubMed] [Google Scholar]



