Abstract
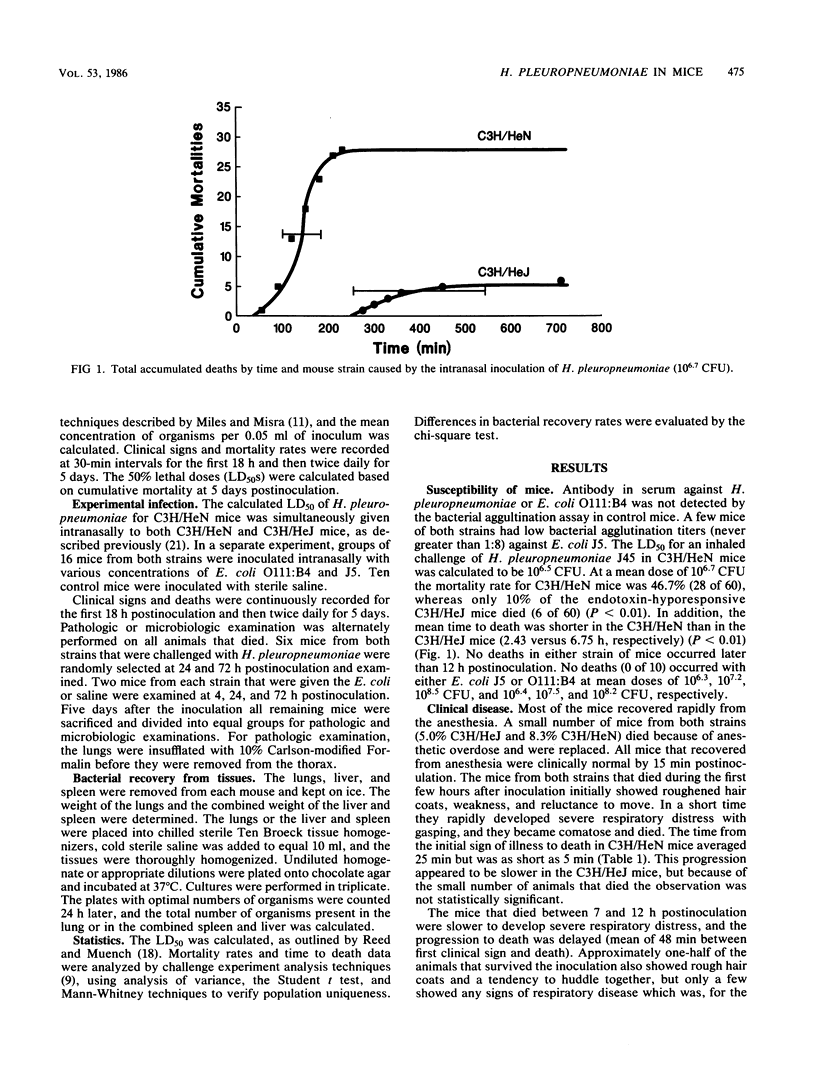
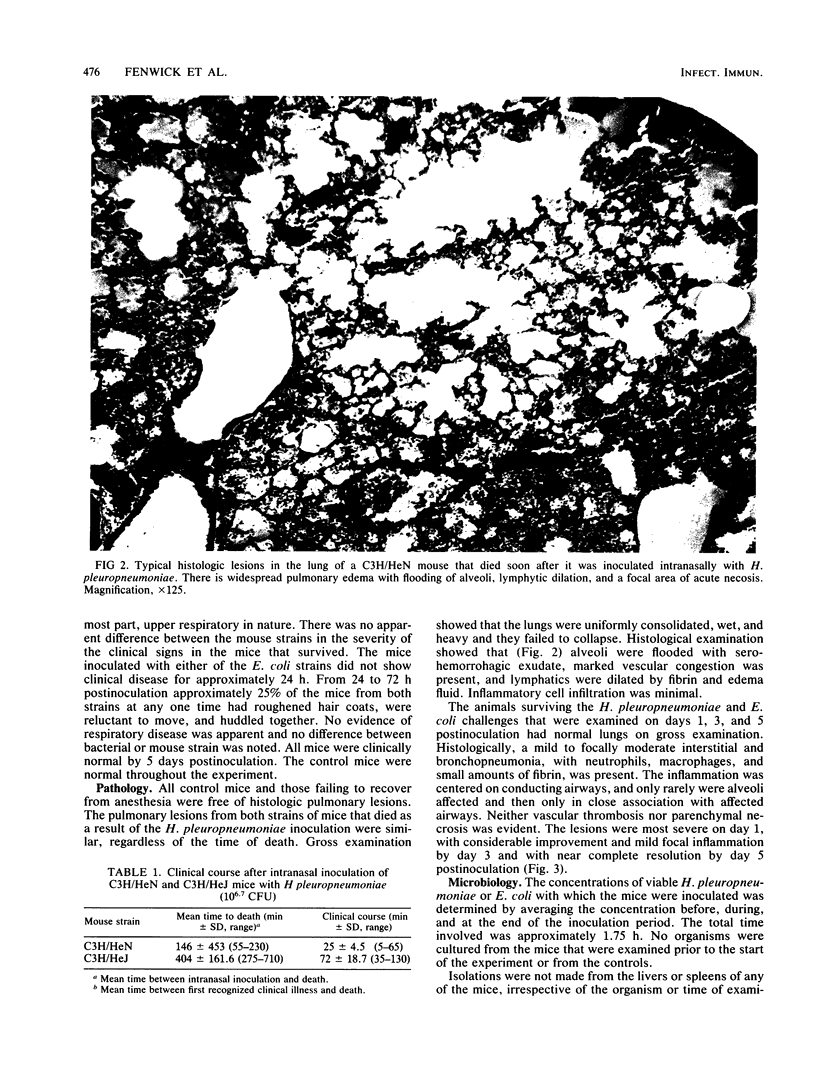
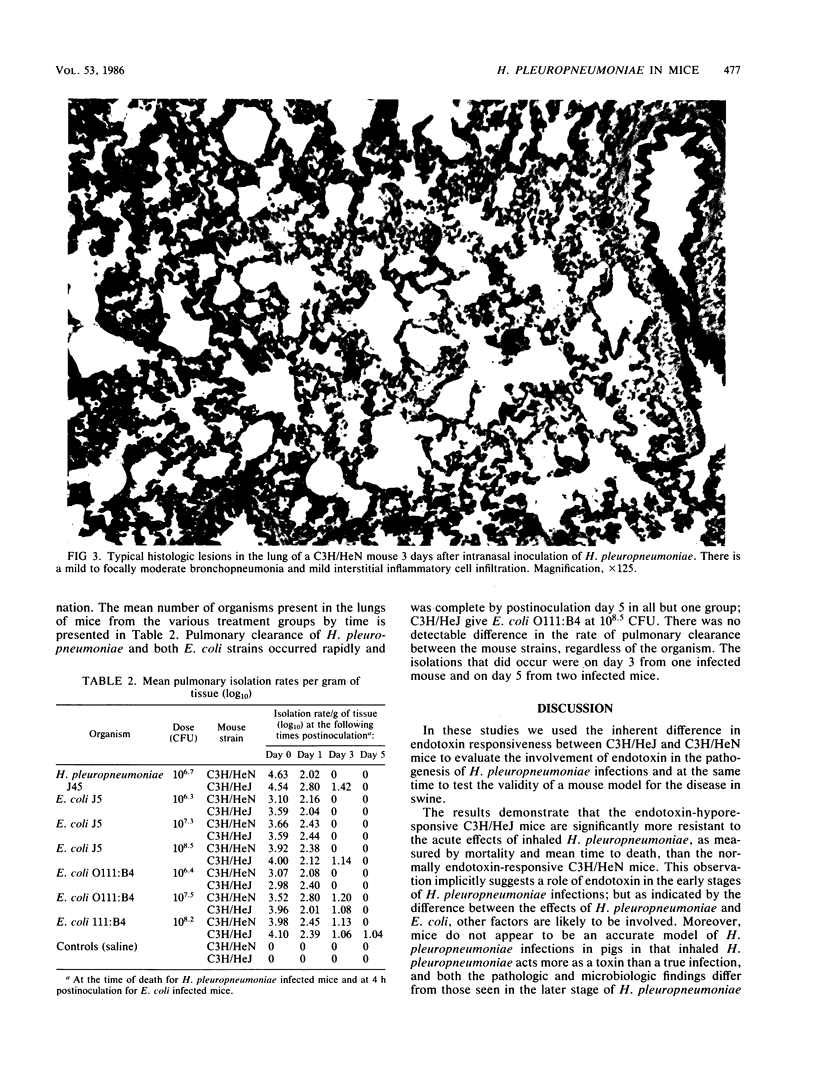
Comparisons were made in the mortality associated with an inhaled dose of viable Haemophilus pleuropneumoniae type 5, strain J45, between adult C3H/HeN and C3H/HeJ mice. Mice of both strains were also challenged with Escherichia coli strains O111:B4 and J5. The 50% lethal dose (LD50) of H. pleuropneumoniae in C3H/HeN mice was calculated to be 10(6.5) CFU. At a mean dose of 10(6.7) CFU a 46% mortality rate occurred in C3H/HeN mice, whereas only 10% of the C3H/HeJ mice died (P less than 0.01). Deaths occurred significantly earlier in C3H/HeN mice (P less than 0.01). No deaths occurred later than 12 h postinfection in either group. Pulmonary lesions in the mice that died were similar to those in pigs that die during the acute phase of H. pleuropneumoniae infection. In surviving mice of both strains, a mild resolving interstitial and bronchopneumonia was present which was not typical of subacute H. pleuropneumoniae infections in swine. Quantitative bacterial isolations from the lungs, liver, and spleen indicate that H. pleuropneumoniae did not multiply in the lungs, was rapidly cleared, and did not become systemic. No deaths occurred in the mice inoculated with E. coli J5 or O111:B4 at mean doses of 10(6.3), 10(7.2), and 10(8.5) CFU, and 10(6.4), 10(7.5), and 10(8.2) CFU, respectively. The difference in the mortality rate between the C3H/HeN and C3H/HeJ mice suggests that endotoxin may be involved in acute deaths in pigs infected with H. pleuropneumonia. As indicated by the E. coli challenge, however, other factors are also likely to be involved. Because of the differences in the pathology and microbiology following H. pleuropneumoniae pulmonary infections in mice and pigs, mice do not appear to be an accurate model of the overall disease in swine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendixen P. H., Shewen P. E., Rosendal S., Wilkie B. N. Toxicity of Haemophilus pleuropneumoniae for porcine lung macrophages, peripheral blood monocytes, and testicular cells. Infect Immun. 1981 Sep;33(3):673–676. doi: 10.1128/iai.33.3.673-676.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Deakins L. W., Killar L., Saluk P. H., Sultzer B. M. Dissociation of innate susceptibility to Salmonella infection and endotoxin responsiveness in C3HeB/FeJ mice and other strains in the C3H lineage. Infect Immun. 1982 May;36(2):696–703. doi: 10.1128/iai.36.2.696-703.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick B. W., Osburn B. I., Olander H. J. Isolation and biological characterization of two lipopolysaccharides and a capsular-enriched polysaccharide preparation from Haemophilus pleuropneumoniae. Am J Vet Res. 1986 Jul;47(7):1433–1441. [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., McGhee J. R., Michalek S. M., Svanborg Edén C. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun. 1984 Dec;46(3):839–844. doi: 10.1128/iai.46.3.839-844.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Tanner A. C., Socransky S. S. Morphology and ultrastructure of oral strains of Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus. Infect Immun. 1980 Nov;30(2):588–600. doi: 10.1128/iai.30.2.588-600.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K., Nakai T., Sawata A. Interaction between heat-stable hemolytic substance from Haemophilus pleuropneumoniae and porcine pulmonary macrophages in vitro. Infect Immun. 1986 Feb;51(2):563–570. doi: 10.1128/iai.51.2.563-570.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell F. D. Evaluation of survival in challenge experiments. Microbiol Rev. 1978 Mar;42(1):237–249. doi: 10.1128/mr.42.1.237-249.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Structural and antigenic heterogeneity of lipopolysaccharides of Campylobacter jejuni and Campylobacter coli. Infect Immun. 1984 Jul;45(1):210–216. doi: 10.1128/iai.45.1.210-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal K. R., Higgins R., Larivière S., Leblanc D. A 2-mercaptoethanol tube agglutination test for diagnosis of Haemophilus pleuropneumoniae infection in pigs. Am J Vet Res. 1984 Apr;45(4):715–719. [PubMed] [Google Scholar]
- Morrison D. C., Cochrane C. G. Direct evidence for Hageman factor (factor XII) activation by bacterial lipopolysaccharides (endotoxins). J Exp Med. 1974 Sep 1;140(3):797–811. doi: 10.1084/jem.140.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R., Mandrup M. Pleuropneumonia in swine caused by Haemophilus parahaemolyticus. A study of the epidemiology of the infection. Nord Vet Med. 1977 Nov;29(11):465–473. [PubMed] [Google Scholar]
- O'Brien A. D., Rosenstreich D. L., Scher I., Campbell G. H., MacDermott R. P., Formal S. B. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J Immunol. 1980 Jan;124(1):20–24. [PubMed] [Google Scholar]
- Perfumo C. J., Rehbinder C., Karlsson K. Swine pleuropneumonia produced by Haemophilus pleuropneumoniae. III. An electron microscopic study. Zentralbl Veterinarmed B. 1983 Oct;30(9):678–684. doi: 10.1111/j.1439-0450.1983.tb01893.x. [DOI] [PubMed] [Google Scholar]
- Robson H. G., Vas S. I. Resistance of inbred mice to Salmonella typhimurium. J Infect Dis. 1972 Oct;126(4):378–386. doi: 10.1093/infdis/126.4.378. [DOI] [PubMed] [Google Scholar]
- Rosendal S., Mitchell W. R. Epidemiology of Haemophilus pleuropneumoniae infection in pigs: a survey of Ontario Pork Producers, 1981. Can J Comp Med. 1983 Jan;47(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Rushton B. Induction of pneumonia in mice with Pasteurella haemolytica. J Comp Pathol. 1978 Jul;88(3):477–480. doi: 10.1016/0021-9975(78)90053-1. [DOI] [PubMed] [Google Scholar]
- Saunders J. R., Osborne A. D., K-Sebunya T. Pneumonia in Saskatchewan swine: abattoir incidence of intrathoracic lesions in pigs from a herd infected with Haemophilus pleuropneumoniae and from other herds. Can Vet J. 1981 Aug;22(8):244–247. [PMC free article] [PubMed] [Google Scholar]
- Schultz R. A., Young T. F., Ross R. F., Jeske D. R. Prevalence of antibodies to Haemophilus pleuropneumoniae in Iowa swine. Am J Vet Res. 1982 Oct;43(10):1848–1851. [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R., Osborne A. D. Dose response relationship of Haemophilus pleuropneumoniae aerosols in pigs. Can J Comp Med. 1983 Jan;47(1):54–56. [PMC free article] [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Pulmonary clearance of Haemophilus pleuropneumoniae and Serratia marcescens in mice. Am J Vet Res. 1982 Oct;43(10):1799–1801. [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Studies on immunity to Haemophilus pleuropneumoniae infections in mice. Am J Vet Res. 1982 Oct;43(10):1793–1798. [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of leucocyte responses to endotoxin. Nature. 1968 Sep 21;219(5160):1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- Watson J., Largen M., McAdam K. P. Genetic control of endotoxic responses in mice. J Exp Med. 1978 Jan 1;147(1):39–49. doi: 10.1084/jem.147.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Riblet R., Taylor B. A. The response of recombinant inbred strains of mice to bacterial lipopolysaccharides. J Immunol. 1977 Jun;118(6):2088–2093. [PubMed] [Google Scholar]




