Abstract
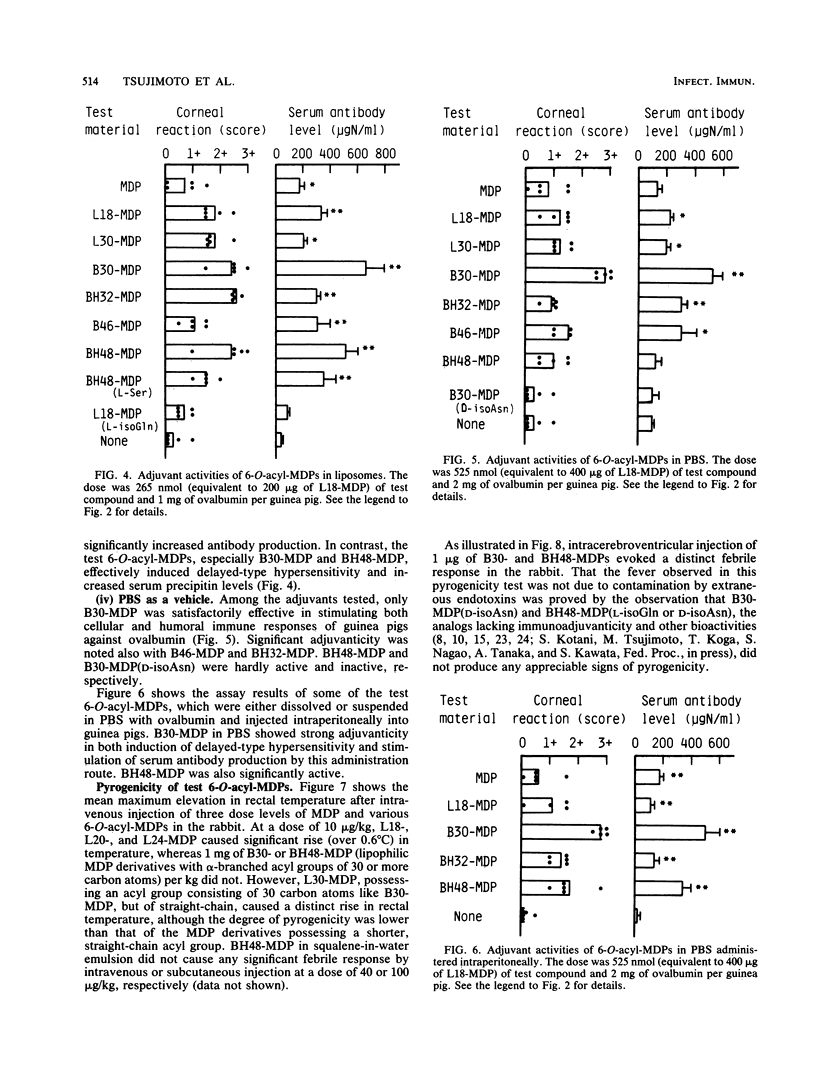
Thirteen 6-O-acyl-N-acetylmuramyl-L-alanyl-D-isoglutamines (6-O-acyl-MDPs), including four inactive D-isoasparagine and L-isoglutamine analogs, were tested for their pyrogenicity and immunopotentiating activity to stimulate primary humoral and cellular immune responses in guinea pigs to a model protein antigen, ovalbumin, when administered in various vehicles. Among them, derivatives whose muramic acid residue was substituted by alpha-branched (and beta-hydroxylated) higher fatty acids at the carbon-6 position, especially 6-O-(2-tetradecylhexadecanoyl)-MDP (B3O-MDP) and, to a lesser extent, 6-O-(3-hydroxy-2-docosylhexacosanoyl)-MDP (BH48-MDP) and its L-serine analog [BH48-MDP(L-Ser)], were found to exert strong adjuvant activity in both the induction of delayed-type hypersensitivity and the stimulation of circulating precipitating antibody levels when combined with nonirritating vehicles (liposomes, squalene-in-water emulsion, and phosphate-buffered saline). These vehicles did not efficiently support the adjuvant activity of MDP, the parent molecule of the above lipophilic derivatives. Pyrogenicity tests showed that introduction of alpha-branched higher fatty acid groups but not of straight, long-chain fatty acids at the 6-position of the muramic acid residue resulted in marked decrease of the pyrogenicity inherent to MDP via intravenous administration.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Lederer E. Muramyl peptides: immunomodulators, sleep factors, and vitamins. Med Res Rev. 1984 Apr-Jun;4(2):111–152. doi: 10.1002/med.2610040202. [DOI] [PubMed] [Google Scholar]
- Audibert F., Chédid L., Lefrancier P., Choay J. Distinctive adjuvanticity of synthetic analogs of mycobacterial water-soluble components. Cell Immunol. 1976 Feb;21(2):243–249. doi: 10.1016/0008-8749(76)90053-8. [DOI] [PubMed] [Google Scholar]
- Audibert F., Parant M., Damais C., Lefrancier P., Derrien M., Choay J., Chedid L. Dissociation of immunostimulant activities of muramyl dipeptide (MDP) by linking amino-acids or peptides to the glutaminyl residue. Biochem Biophys Res Commun. 1980 Sep 30;96(2):915–923. doi: 10.1016/0006-291x(80)91442-4. [DOI] [PubMed] [Google Scholar]
- Chedid L., Audibert F., Johnson A. G. Biological activities of muramyl dipeptide, a synthetic glycopeptide analogous to bacterial immunoregulating agents. Prog Allergy. 1978;25:63–105. [PubMed] [Google Scholar]
- Chedid L., Audibert F., Lefrancier P., Choay J., Lederer E. Modulation of the immune response by a synthetic adjuvant and analogs. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2472–2475. doi: 10.1073/pnas.73.7.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Emori K., Nagao S., Shigematsu N., Kotani S., Tsujimoto M., Shiba T., Kusumoto S., Tanaka A. Granuloma formation by muramyl dipeptide associated with branched fatty acids, a structure probably essential for tubercle formation by Mycobacterium tuberculosis. Infect Immun. 1985 Jul;49(1):244–249. doi: 10.1128/iai.49.1.244-249.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta H., Sanchez L. F., Takada H., Homma M., Kotani S. Enhancement of dengue virus infection in cultured mouse macrophages by lipophilic derivatives of muramyl peptides. Microbiol Immunol. 1985;29(6):533–541. doi: 10.1111/j.1348-0421.1985.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Kotani S., Kinoshita F., Morisaki I., Shimono T., Okunaga T., Takada H., Tsujimoto M., Watanabe Y., Kato K., Shiba T. Immunoadjuvant activities of synthetic 6-O-acyl-N-acetylmuramyl-L-alanyl-D-isoglutamine with special reference to the effect of its administration with liposomes. Biken J. 1977 Dec;20(3-4):95–103. [PubMed] [Google Scholar]
- Kotani S., Kinoshita F., Watanabe Y., Morisaki I., Shimono T., Kato K., Shiba T., Kusumoto S., Ikenaka K., Tarumi Y. Effects of chemical modifications of the glutamic acid residue in N-acetylmuramyl peptides on the immunoadjuvancies of the molecules. Biken J. 1977 Dec;20(3-4):125–130. [PubMed] [Google Scholar]
- Kotani S., Narita T., Stewart-Tull D. E., Shimono T., Watanabe Y. Immunoadjuvant activities of cell walls and their water-soluble fractions prepared from various gram-positive bacteria. Biken J. 1975 Jun;18(2):77–92. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Morisaki I., Kato K. The effect of replacement of L-alanine residue by glycine, L-serine or D-alanine in an N-acetylmuramyl-L-alanyl-D-isoglutamine on immunoadjuvancies of molecules. Biken J. 1977 Jun;20(2):39–45. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Shimono T., Morisaki I. Immunoadjuvant activities of synthetic N-acetyl-muramyl-peptides or -amino acids. Biken J. 1975 Jun;18(2):105–111. [PubMed] [Google Scholar]
- Ogawa T., Kotani S., Tsujimoto M., Kusumoto S., Shiba T., Kawata S., Yokogawa K. Contractile effects of bacterial cell walls, their enzymatic digests, and muramyl dipeptides on ileal strips from guinea pigs. Infect Immun. 1982 Feb;35(2):612–619. doi: 10.1128/iai.35.2.612-619.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Cummings N. P., Shiba T., Kusumoto S., Kotani S. Lipophilic derivative of muramyl dipeptide is more active than muramyl dipeptide in priming macrophages to release superoxide anion. Infect Immun. 1980 Aug;29(2):617–622. doi: 10.1128/iai.29.2.617-622.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Sutcliffe J. G., Green N., Lerner R. A. Synthetic peptide immunogens as vaccines. Annu Rev Microbiol. 1983;37:425–446. doi: 10.1146/annurev.mi.37.100183.002233. [DOI] [PubMed] [Google Scholar]
- Stewart-Tull D. E. Immunopotentiating conjugates. Vaccine. 1985 Mar;3(1):40–44. doi: 10.1016/0264-410x(85)90010-6. [DOI] [PubMed] [Google Scholar]
- Stewart-Tull D. E., Shimono T., Kotani S., Kato M., Ogawa Y., Yamamura Y., Koga T., Pearson C. M. The adjuvant activity of a non-toxic, water-soluble glycopeptide present in large quantities in the culture filtrate of Mycobacterium tuberculosis strain DT. Immunology. 1975 Jul;29(1):1–15. [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G., Shinnick T. M., Green N., Lerner R. A. Antibodies that react with predetermined sites on proteins. Science. 1983 Feb 11;219(4585):660–666. doi: 10.1126/science.6186024. [DOI] [PubMed] [Google Scholar]
- Yarkoni E., Rapp H. J. Influence of type of oil and surfactant concentration on the efficacy of emulsified Mycobacterium bovis BCG cell walls to induce tumor regression in guinea pigs. Infect Immun. 1980 Jun;28(3):881–886. doi: 10.1128/iai.28.3.881-886.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni E., Rapp H. J. Tumor regression after intralesional injection of mycobacterial components emulsified in 2,6,10,15,19,23-hexamethyl-2,6,10,14,18,22-tetracosahexaene (squalene), 2,6,10,15,19,23-hexamethyltetracosane (squalane), peanut oil, or mineral oil. Cancer Res. 1979 May;39(5):1518–1520. [PubMed] [Google Scholar]


