Abstract
Recent advances in understanding β-catenin-independent WNT (non-canonical) signalling suggest an increasing complexity, raising the question of how individual non-canonical pathways are induced and regulated. Here, we examine whether intracellular signalling components such as β-arrestin (β-arr) and casein kinases 1 and 2 (CK1 and CK2) can contribute to determining signalling specificity in β-catenin-independent WNT signalling to the small GTPase RAC-1. Our findings indicate that β-arr is sufficient and required for WNT/RAC-1 signalling, and that casein kinases act as a switch that prevents the activation of RAC-1 and promotes other non-canonical WNT pathways through the phosphorylation of dishevelled (DVL, xDSH in Xenopus). Thus, our results indicate that the balance between β-arr and CK1/2 determines whether WNT/RAC-1 or other non-canonical WNT pathways are activated.
Keywords: convergent extension movements, RAC-1, RHO-like GTPases, Xenopus laevis, WNT-5A
Introduction
WNTs are secreted glycolipoproteins that activate β-catenin-dependent and -independent (non-canonical) pathways. Several recent reports have provided evidence for the complexity of the non-canonical WNT pathways (Semenov et al, 2007). The individual β-catenin-independent WNT branches, which mediate planar cell polarity (for a list of abbreviations, see supplementary information online) or convergent extension movements, are so diverse that it has become necessary to name them by the signalling components involved. Here, we refer to the following pathways as defined in the original studies: WNT/RHO (Habas et al, 2003), WNT/RAC-1 (Habas et al, 2003), WNT/Ca2+ (Kühl et al, 2001), WNT/ROR2/CDC42 (Schambony & Wedlich, 2007) and WNT/CK1/RAP1 (Tsai et al, 2007). β-Arrestin (β-arr) was recently found to regulate WNT/β-catenin signalling (Bryja et al, 2007b) and convergent extension movements in Xenopus laevis through RHO-A (Kim & Han, 2007). The frequent involvement of β-arr in WNT signalling prompted us to examine whether it mediates the effects of WNTs on other branches of non-canonical signalling.
We found that β-arr was required for WNT/RAC-1 signalling, but was not essential for the WNT/ROR2/CDC42 pathway. Furthermore, in vitro and in vivo data support that CK1/2 prevent the activation of RAC-1, but promote the formation of phosphorylated and shifted dishevelled (PS-DVL; dishevelled, xDSH in Xenopus). This indicates that the recruitment of β-arr to DVL selects and is required for WNT/RAC-1 signalling, and that casein kinases switch signalling from WNT/RAC-1 towards non-canonical pathways mediated by PS-DVL.
Results And Discussion
WNT-induced activation of RAC-1 requires β-arr
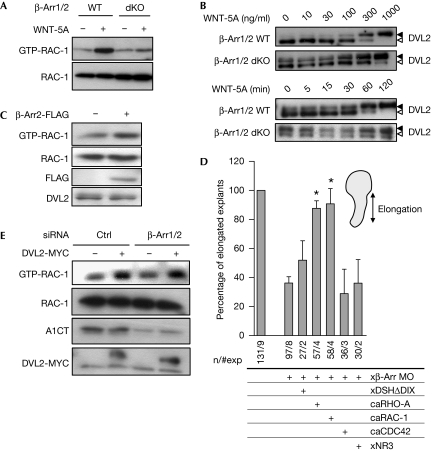
First, we examined the activation of small GTPases by WNT-5A in mouse embryonic fibroblasts (MEFs) using GTPase pull-down assays. Although WNT-5A, which does not affect β-catenin signalling (supplementary Fig S1A online), activated RAC-1 (Fig 1A; supplementary Fig S1B online; 4.1-fold±0.8 standard error of the mean, s.e.m.), it activated neither RHO-A nor CDC42 (supplementary Fig S1C online). Interestingly, the activation of RAC-1 by WNT-5A was blocked in MEFs lacking β-arr1 and β-arr2 (β-arr1/2 double knock out (dKO)), arguing that β-arr is required for WNT/RAC-1 signalling. In the same cells, WNT-5A-induced formation of PS-DVL (González-Sancho et al, 2004; Bryja et al, 2007c) was less efficient and delayed compared with wild-type MEFs (Fig 1B). It is important to note that although the lack of β-arr interferes with WNT-induced dynamics of PS-DVL, it increases the basal PS-DVL (Fig 1B). This suggests that β-arr is not necessary for PS-DVL but modulates its formation. Re-transfection of β-arr1 in dKO MEFs reduces the basal PS-DVL and restores the WNT-5A-induced activation of RAC-1 (supplementary Fig S1D online). To analyse whether β-arr is sufficient for the activation of RAC-1, we overexpressed β-arr2-FLAG in MEFs and observed increased activation of RAC-1 (Fig 1C; supplementary Fig S1E online). To confirm these results, we overexpressed β-arr2-FLAG and downregulated xβ-arr by using antisense morpholinos (Xβ-arr MO; Bryja et al, 2007b) in X. laevis embryos. We found that overexpression of β-arr increased basal RAC-1 activity and that it was decreased on downregulation (supplementary Fig S1F online). Furthermore, the effect of the xβ-arr MO could be reversed by overexpression of MO-insensitive, murine β-arr, emphasizing the specificity of the xβ-arr MO. These findings identify β-arr as a signalling component required for the regulation of RAC-1 in vitro and in vivo. For functional analysis, we performed Keller open-face explant elongation assays in X. laevis embryos, an established measure of convergent extension (Keller et al, 2003; Schambony & Wedlich, 2007). Both overexpression and downregulation of β-arr modulated convergent extension, and the latter was reversed by co-injection of the MO-insensitive haemagglutinin (HA)-β-arr (supplementary Fig S1F online), confirming previous results (Kim & Han, 2007). Kim & Han (2007) showed that β-arr2 regulates convergent extension movements in X. laevis through RHO-A. Thus, our results, combined with those reported by Kim & Han, suggest that various subsets of RHO family GTPases are regulated by β-arr.
Figure 1.
β-Arrestin is necessary for WNT-induced activation of RAC-1. (A) WNT-5A induced the activation of RAC-1 (GTP-RAC-1) in wild-type MEFs but not in MEFs lacking β-arrestin (β-arr1/2 double knock out (dKO)). One representative experiment is shown; in total, three are summarized in supplementary Fig S1B online. (B) WNT-5A-induced formation of phosphorylated and shifted (DVL2, open arrowhead; PS-DVL2, filled arrowhead) in a dose- and time-dependent manner in wild-type and β-arr1/2dKO MEFs is shown. (C) The activation and expression of RAC-1 were monitored in MEFs overexpressing β-arr2-FLAG. (D) Downregulation of β-arr is compensated by constitutively active RAC-1 and RHO-A. Keller open-face explants were scored for elongation on injection with Xβ-arr MO and indicated RNAs. Asterisks indicate values that are significantly different (P>0.95, t-test) from xβ-arr MO. (E) Downregulation of β-arr1/2 expression by short interfering RNA (siRNA) in HEK293 cells does not affect the DVL2-MYC- induced activation of RAC-1 (GTP-RAC-1). Levels of β-arr are detected by the A1CT antibody. ca, constitutively active; DVL/DSH, dishevelled; HEK, human embryonic kidney; MEF, mouse embryonic fibroblast; MO, morpholino; WT, wild type.
β-Arr acts upstream from RAC-1 in convergent extension
To examine the regulation of RAC-1 by β-arr, we analysed the effect of the following RNAs encoding for WNT signalling components on the convergent extension phenotype in β-arr knockdowns: xDSHΔDIX (a mutant lacking the DIX domain), a constitutively active WNT/planar cell polarity DVL mutant, constitutively active caRHO-A, caRAC-1 or caCDC42, and xNR3, a WNT/β-catenin transcriptional target (Fig 1D; Kühl et al, 2001; Bryja et al, 2007b). Both RAC-1 and RHO-A compensated for the lack of β-arr, and xDSHΔDIX partly restored explant elongation, whereas CDC42 and xNR3 had no effect (Fig 1D). Complementary results from β-arr1/2 short interfering RNA (siRNA) experiments indicate that β-arr is not required for the DVL-induced activation of RAC-1 (Fig 1E). Other epistatic experiments showed that RHO-A and RAC-1 act downstream from xDSH in the regulation of convergent extension (supplementary Fig S1H online). When discussing the WNT-induced β-arr-dependent formation of PS-DVL, it is worthwhile mentioning that WNT-3A does not induce the activation of RAC-1 in MEFs (supplementary Fig S1I online), despite the fact that it induces β-arr-dependent PS-DVL (Bryja et al, 2007b) with a similar time course (Bryja et al, 2007c).
Taken together, these results indicate that: β-arr acts upstream or on the level of DVL; RHO-A and RAC-1 but not CDC42 are mediators downstream from β-arr and DVL; and the WNT/β-catenin pathway is not involved in the observed phenotype.
β-Arr is not required for xWNT-5A/ROR signalling
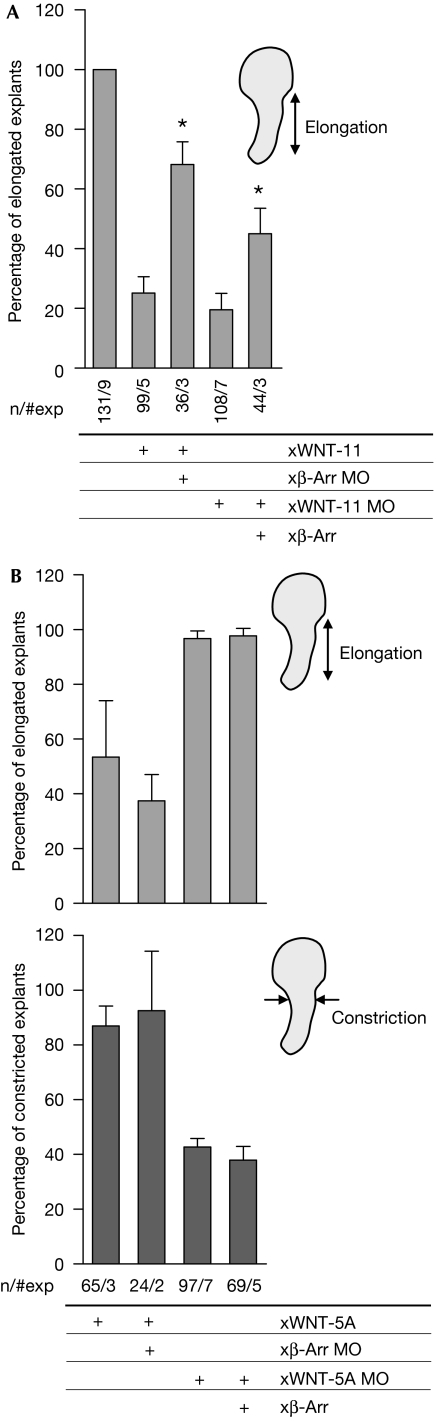
It has been shown previously that WNT-regulated convergent extension in X.laevis Keller explants is mediated by several distinct molecular mechanisms (Tada et al, 2002). Although xWNT-11 exerts its effects on convergent extension by activation of the WNT/RAC-1, WNT/RHO and WNT/FZD7/Ca2+ pathways (Winklbauer et al, 2001; Habas et al, 2003), xWNT-5A is not required for Keller explant elongation but instead for explant constriction, and activates the WNT/ROR2/CDC42 pathway (Unterseher et al, 2004; Schambony & Wedlich, 2007). To examine the function of β-arr for xWNT-11 or xWNT-5A signals, we examined whether β-arr mediated their effects in Keller explants. Strikingly, only xWNT-11 (Fig 2A), but not xWNT-5A (Fig 2B), was reversed by Xβ-arr MO co-injection. Consistently, the negative effects of xWNT-11 MO on explant elongation were partly reversed by the overexpression of β-arr (Fig 2A); however, β-arr did not restore constriction in xWNT-5A-depleted explants (Fig 2B), arguing that β-arr is not involved in the xWNT-5A/ROR2 pathway.
Figure 2.
β-Arrestin mediates WNT-11- but not WNT-5A-induced gastrulation movements in Xenopus laevis embryos. Keller open-face explant elongation (A,B, upper panels) and constriction (B, lower panel) were scored in X. laevis embryos injected with indicated RNAs and morpholinos (MOs). Asterisks indicate values that are significantly different (P>0.95, t-test) from xWNT-11 and xWNT-11 MO.
To relate the xWNT-11-induced pathway to the small GTPases, we examined whether caRHO-A, caRAC-1 and caCDC42 or dnRHO-A and dnRAC-1 (supplementary Fig S2A online) rescued the effects of xWNT-11 MO or xWNT-11, respectively. Reduced explant elongation in xWNT-11-depleted explants was partly rescued by coexpression of caRHO-A and caRAC-1 (but not caCDC42), and xWNT-11 overexpression was compensated by dnRHO-A, dnRAC-1 and xDSHΔDEP, a mutant lacking the RAC-1-activating DEP domain (supplementary Fig S2B online; Habas et al, 2003). These experiments confirmed the crucial function of RAC-1 and RHO-A downstream from xWNT-11 in convergent extension movements and indicated that xWNT-11 signalling to RHO/RAC also includes xDSH.
CK1/2 as switches in non-canonical WNT signalling
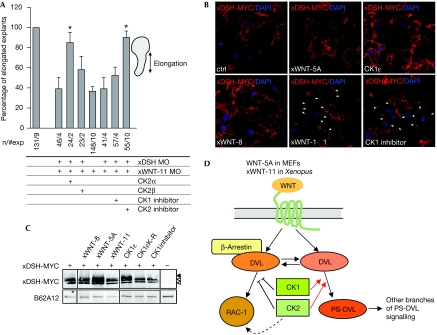
It is known that WNTs directly activate at least two kinases: casein kinase (CK)1 and CK2 (Willert et al, 1997; Swiatek et al, 2004; Gao & Wang, 2006; Bryja et al, 2007d), which, in turn, phosphorylate DVL to form PS-DVL. In line with these findings, it has been shown previously that CK1δ/ɛ are crucial components of the β-catenin-independent WNT signalling machinery (McKay et al, 2001; Klein et al, 2006; Strutt et al, 2006; Bryja et al, 2007d). Furthermore, it was recently shown that CK1ɛ acts through the small GTPases RAP1 (Tsai et al, 2007). Interestingly, CK1 and CK2 can physically interact with β-arr (Xiao et al, 2007), and we have already shown that CK1 mediates communication between β-arr and DVL in the WNT/β-catenin pathway (Bryja et al, 2007b). To test whether PS-DVL and CK1 have a similar function in β-arr-dependent WNT/RAC-1 signalling, we inhibited CK1 pharmacologically with D4476 (4-[4-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-5-pyridin-2-yl-1H-imidazol-2-yl]benzamide). As expected, D4476 led to a reduction of basal PS-DVL2 levels but, surprisingly, it increased the activation of RAC-1 (Fig 3A). Similar results were obtained with the inhibition of CK2 by TBBt (4,5,6,7-tetrabromo-2-azabenzotriazole; Fig 3A). The presence of the DVL protein per se seems to be required for both the activation of RAC-1 and the maintenance of RAC-1 expression levels (supplementary Fig S3A online). Thus, DVL as a protein, but not PS-DVL, which is dependent on CK1 and CK2, is required for the activation of RAC-1. In summary, our data suggest that CK1 and CK2, and possibly also PS-DVL, negatively regulate WNT/RAC-1 signalling in vitro.
Figure 3.
Casein kinases 1 and 2 differentially regulate the formation of PS-DVL and the activation of RAC-1. (A) The activation of RAC-1 (GTP-RAC-1), and the expression levels of RAC-1 and DVL2/PS-DVL2 (DVL2, open arrowhead; PS-DVL2, filled arrowhead) in MEFs were monitored in the absence (−) or presence (+) of the CK1 (100 μM) or CK2 inhibitor (100 μM). (B) HEK293 cells were transfected with RAC-1-MYC in the presence of DVL3-FLAG and/or CK1ɛ. RAC-1-MYC activity (GTP-RAC-1) was determined by pull down, and RAC-1-MYC, DVL3-FLAG, CK1ɛ and actin levels were determined in cell lysates. Note the CK1ɛ-induced mobility shift of DVL3-FLAG. (C,D) Explant elongation after downregulation of β-arrestin (C; xβ-arr MO) or xWNT-11 (D; xWNT-11 MO), in combination with CK1ɛ, CK2α, CK2β and CK2α/β, or CK1 and CK2 inhibitors, was evaluated. Asterisks indicate values that are significantly different (P>0.95, t-test) from (C) xβ-arr MO and (D) xWNT-11 MO. (E) Effect of xWNT-11 MO on the activation of RAC-1 in the absence (−) and presence (+) of CK1 or CK2 inhibitors was measured by active GTP-RAC-1 pull down from Xenopus embryo lysates. Levels of RAC-1 in the lysates are shown in the lower panel. CK, casein kinase; HEK, human embryonic kidney; MEF, mouse embryonic fibroblast; MO, morpholino; PS-DVL, phosphorylated and shifted dishevelled.
Importantly, WNTs seem to concomitantly evoke the activation of RAC-1/RHO-A (Habas et al, 2003; Fig 1A) and the formation of PS-DVL (González-Sancho et al, 2004; Schulte et al, 2005; Bryja et al, 2007d; Fig 1B), which depend on CK1 (Bryja et al, 2007d) and CK2 (Gao & Wang, 2006; Fig 3A). Interestingly, PS-DVL is usually formed 30–45 min after stimulation with WNT-5A (Bryja et al, 2007d; Fig 1B), whereas RAC-1 was shown to be already activated 5 min after stimulation (Kurayoshi et al, 2006), raising the possibility that the activation of RAC-1 precedes the formation of PS-DVL and activation of other branches of non-canonical signalling. Can CK1/CK2 thus act as a switch between individual β-catenin-independent WNT pathways at the level of DVL? To test this possibility, we induced the activation of RAC-1 directly by DVL3 and studied the effects of CK1 on this process (Fig 3B). Although overexpression of DVL3 increased the activation of RAC-1, this positive effect on RAC-1 was abolished when the formation of PS-DVL3 was induced by simultaneous CK1ɛ co-expression. Furthermore, we found, by using the AP-1 reporter assay, that basal and DVL3––but not phorbolester––or ca-RAC-1-induced AP-1 activity was enhanced by treatment with D4476 (supplementary Fig S3B online). These data suggest that: PS-DVL (mediated by CK1/CK2 activity) does not participate in the WNT/β-arr/RAC-1/AP-1 pathway; and that the activation of CK1/CK2 and formation of PS-DVL leads to a change in the signalling function of DVL, displacing it from the RAC-1 pathway.
CK 1 and 2 in the regulation of convergent extension
In vivo, we confirmed that both CK1 and CK2 are involved in and required for the regulation of convergent extension movements in Keller explants (supplementary Fig S3C online). However, we observed differences in the ability of CK1 and CK2 to restore elongation in xβ-arr or xWNT-11-depleted explants. xβ-arr MO was not rescued by CK1ɛ, CK2α or CK2 holoenzyme and only partly by CK2β (Fig 3C). By contrast, CK2α, CK2β and CK2 holoenzyme, but not CK1ɛ rescued xWNT-11 depletion (Fig 3D). Consistently, xWNT-11 overexpression was rescued by dominant negative (dn) CK2α, but not by dnCK1ɛ (supplementary Fig S3D online). Furthermore, in agreement with our biochemical data, chemical inhibition of CK1 or CK2 restored explant elongation (Fig 3D) and RAC-1 activity (Fig 3E) in xWNT-11 MO-injected explants. Simultaneous downregulation of xWNT-11 and xDSH (for optimizing xDSH MOs, see supplementary Fig S4A online) resulted in an additive phenotype that was rescued by co-injection of CK2α, but not CK2β (Fig 4A). Overall, these results indicate that in vivo CK1ɛ and CK2α do not positively regulate Xβ-arr-dependent branches of non-canonical signalling, and that xWNT-11 activates not only β-arr/xDSH/RAC-1 signalling but also other pathways that involve CK2, most likely WNT/Ca2+ signalling. This assumption is supported by the observation that CK2α rescues convergent extension in PTX-treated explants (unpublished observation).
Figure 4.
DVL-dependent and -independent function of casein kinases. (A) Convergent extension was quantified in embryos treated with both xDSH MO and xWNT-11 MO in combination with overexpression of CK2α, CK2β or CK1, CK2 inhibition. Elongation of Keller explants was quantified. Asterisks indicate values that are significantly different (P>0.95, t-test) from xWNT-11 MO/xDSH MO. (B) Immunocytochemical localization of xDSH-MYC (nuclear counterstain DAPI) in Xenopus mesoderm in control (ctrl), WNT- or CK1ɛ-overexpressing or D4476-treated embryos. Arrowheads point at membraneous xDSH-MYC. (C) Corresponding biochemical data. xDSH-MYC was detected in embryo lysates and analysed for the expression of xDSH-MYC (xDSH, open arrowhead; PS-xDSH, filled arrowhead). The antigen B62A12 was used as a loading control. (D) Schematic view of the role of casein kinases and β-arrestin in the regulation of formation of PS-DVL and WNT-induced signalling to RAC-1; for details, see text. CE, convergent extension; CK, casein kinase; DAPI, 4,6-diamidino-2-phenylindole; DVL/DSH, dishevelled; MO, morpholino; PS-DVL, phosphorylated and shifted DVL.
Interestingly, the positive effect of inhibition of CK1 on convergent extension was strictly xDSH dependent, whereas inhibition of CK2 led to explant elongation (Fig 4A) and the activation of RAC-1 (supplementary Fig S4B online) even after knockdown of xWNT-11/xDSH. To address the requirement of DVL for the CK-modulated activation of RAC-1 in mammalian the cells, we used pretreatment with hyperosmolaric sucrose to deplete cells of DVL (Bryja et al, 2007a). Under these conditions, only inhibition of CK2 increased the activation of RAC-1, further supporting the idea that CK2 but not CK1 mediates the activation of RAC-1 at least partly independently of DVL (supplementary Fig S4C online).
When we analysed xDSH subcellular localization (Fig 4B) in response to D4476, we observed that inhibition of CK1 activity resulted in translocation of xDSH-MYC to the cell membrane, similar to the overexpression of xWNT-11, but not of xWNT-5A or xWNT-8, whereas xDSH-MYC was found predominantly in the cytoplasm in CK1ɛ-overexpressing cells. The effect of the treatment was also monitored by the decrease or increase of PS-xDSH, respectively (Fig 4C). This indicates that submembraneous DVL could represent a pool of DVL that is active in the RAC-1 pathway and that is not phosphorylated by CK1/2, but does not form PS-DVL.
In summary, our data first suggest that β-arr is not essential for the WNT/ROR2/CDC42 and non-canonical pathways involving CK1/2 and PS-DVL. However, we found that β-arr is selectively required for the WNT/RAC-1 pathway. This finding selectively links WNT/RAC-1 signalling with other β-arr-regulated pathways and functions, such as receptor endocytosis, internalization and desensitization.
Second, our in vitro and in vivo data support that the formation of PS-DVL (which requires CK1 and/or CK2) and the activation of RAC-1 (which requires inhibition of CK1/2) represent independent events, and potential ways to measure two β-catenin-independent pathways activated by a common ligand (WNT5A or XWNT-11). We propose that CK1 and CK2 can act as a central relay to select the pathways and signalling components being positively and negatively regulated (PS-DVL and RAC-1, respectively, schematized in Fig 4D). Thus, CK1 and CK2 can be considered as switches between individual branches of β-catenin-independent WNT signalling.
Methods
Cell culture, transfection and treatments. Wild-type MEFs and MEFs lacking β-arr 1 and 2 (β-arr1/2dKO) were a gift from Dr Lefkowitz (Kohout et al, 2001). MEFs and human embryonic kidney (HEK) 293 cells were grown in DMEM, 10% FCS, L-glutamine (2 mM), penicillin (50 U/ml) and streptomycin (50 U/ml). Transfection and treatment with D4476, casein kinase 2 inhibitor I (Calbiochem, Nottingham, UK)-TBBt or recombinant mouse WNT-5A (from R&D Systems, Abingdon, UK) were carried out as described previously (Bryja et al, 2007c). For a list of expression vectors, see the supplementary information online.
GTPase pull-down assay and immunoblotting. GST-PAK-CRIB (for glutathione-S-transferase-p21-activated kinase-CDC42/RAC interactive binding domain), GST-WASP-CRIB (WASP for Wiskott–Aldrich syndrome protein) and GST-RHOtekin recombinant proteins were coupled to glutathione sepharose beads for the detection of activated RAC-1, CDC42 and RHO-A, respectively, and the assay performed as described in the supplementary information online. Immunoblotting and sample preparation were carried out as described previously (Bryja et al, 2007c).
Xenopus oocytes and Keller explants. For RNA transcription and oocyte injection, see the supplementary information online. Embryos were cultivated until Nieuwkoop and Faber stage 10.5, and Keller explants were prepared, cultivated and scored as described previously (Unterseher et al, 2004). For inhibitor treatment, D4476 and CK2 inhibitors were added to the medium at 1 μM at the blastula stage (stage 8). xDSH-MYC translocation was examined after injection of 100 pg xDSH-MYC RNA. At onset of gastrulation (stage 10.5), the dorsal marginal zone and the blastocoel roof were prepared and fixed in 4% formalin. Tissue was stained with 9E10 anti-MYC, mounted and examined with a laser scanning confocal microscope.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Fig S1
Acknowledgments
The study was supported by the Swedish Foundation for Strategic Research (INGVAR and Center of Excellence for Developmental Biology), Swedish Research Council, European Union (Eurostemcell), Karolinska Institutet, Åke Wiberg, Signe & Olof Wallenius, Jeanssons, Tore Nilsson Foundations, German Research Foundation (DFG), Ministry of Education and Grant Agency of the Academy of Sciences of the Czech Republic and the European Molecular Biology Organization (for more details, see supplementary information online).
Footnotes
The authors declare that they have no conflict of interest.
References
- Bryja V, Cajánek L, Grahn A, Schulte G (2007a) Inhibition of endocytosis blocks Wnt signalling to β-catenin by promoting dishevelled degradation. Acta Physiol 190: 55–61 [DOI] [PubMed] [Google Scholar]
- Bryja V, Gradl D, Schambony A, Arenas E, Schulte G (2007b) β-Arrestin is a necessary component of Wnt/β-catenin signaling in vitro and in vivo. Proc Natl Acad Sci USA 104: 6690–6695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryja V, Schulte G, Arenas E (2007c) Wnt-3a utilizes a novel low dose and rapid pathway that does not require casein kinase 1-mediated phosphorylation of Dvl to activate β-catenin. Cell Signal 19: 610–616 [DOI] [PubMed] [Google Scholar]
- Bryja V, Schulte G, Rawal N, Grahn A, Arenas E (2007d) Wnt-5a induces dishevelled phosphorylation and dopaminergic differentiation via a CK1-dependent mechanism. J Cell Sci 120: 586–595 [DOI] [PubMed] [Google Scholar]
- Gao Y, Wang HY (2006) Casein kinase 2 is activated and essential for Wnt/β-catenin signaling. J Biol Chem 281: 18394–18400 [DOI] [PubMed] [Google Scholar]
- González-Sancho JM, Brennan KR, Castelo-Soccio LA, Brown AM (2004) Wnt proteins induce dishevelled phosphorylation via an LRP5/6-independent mechanism, irrespective of their ability to stabilize β-catenin. Mol Cell Biol 24: 4757–4768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X (2003) Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev 17: 295–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R, Davidson LA, Shook DR (2003) How we are shaped: the biomechanics of gastrulation. Differentiation 71: 171–205 [DOI] [PubMed] [Google Scholar]
- Kim GH, Han JK (2007) Essential role for β-arrestin 2 in the regulation of Xenopus convergent extension movements. EMBO J 26: 2513–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TJ, Jenny A, Djiane A, Mlodzik M (2006) CKIɛ/discs overgrown promotes both Wnt-Fz/β-catenin and Fz/PCP signaling in Drosophila. Curr Biol 16: 1337–1343 [DOI] [PubMed] [Google Scholar]
- Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ (2001) β-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci USA 98: 1601–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühl M, Geis K, Sheldahl LC, Pukrop T, Moon RT, Wedlich D (2001) Antagonistic regulation of convergent extension movements in Xenopus by Wnt/β-catenin and Wnt/Ca2+ signaling. Mech Dev 106: 61–76 [DOI] [PubMed] [Google Scholar]
- Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A (2006) Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res 66: 10439–10448 [DOI] [PubMed] [Google Scholar]
- McKay RM, Peters JM, Graff JM (2001) The casein kinase I family: roles in morphogenesis. Dev Biol 235: 378–387 [DOI] [PubMed] [Google Scholar]
- Schambony A, Wedlich D (2007) Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell 12: 779–792 [DOI] [PubMed] [Google Scholar]
- Schulte G, Bryja V, Rawal N, Castelo-Branco G, Sousa KM, Arenas E (2005) Purified Wnt-5a increases differentiation of midbrain dopaminergic cells and dishevelled phosphorylation. J Neurochem 92: 1550–1553 [DOI] [PubMed] [Google Scholar]
- Semenov MV, Habas R, Macdonald BT, He X (2007) SnapShot: noncanonical Wnt signaling pathways. Cell 131: 1378. [DOI] [PubMed] [Google Scholar]
- Strutt H, Price MA, Strutt D (2006) Planar polarity is positively regulated by casein kinase Iɛ in Drosophila. Curr Biol 16: 1329–1336 [DOI] [PubMed] [Google Scholar]
- Swiatek W, Tsai IC, Klimowski L, Pepler A, Barnette J, Yost HJ, Virshup DM (2004) Regulation of casein kinase I ɛ activity by Wnt signaling. J Biol Chem 279: 13011–13017 [DOI] [PubMed] [Google Scholar]
- Tada M, Concha ML, Heisenberg CP (2002) Non-canonical Wnt signalling and regulation of gastrulation movements. Semin Cell Dev Biol 13: 251–260 [DOI] [PubMed] [Google Scholar]
- Tsai IC, Amack JD, Gao ZH, Band V, Yost HJ, Virshup DM (2007) A Wnt-CKIvarɛ-Rap1 pathway regulates gastrulation by modulating SIPA1L1, a Rap GTPase activating protein. Dev Cell 12: 335–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterseher F, Hefele JA, Giehl K, De Robertis EM, Wedlich D, Schambony A (2004) Paraxial protocadherin coordinates cell polarity during convergent extension via Rho A and JNK. EMBO J 23: 3259–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brink M, Wodarz A, Varmus H, Nusse R (1997) Casein kinase 2 associates with and phosphorylates dishevelled. EMBO J 16: 3089–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklbauer R, Medina A, Swain RK, Steinbeisser H (2001) Frizzled-7 signalling controls tissue separation during Xenopus gastrulation. Nature 413: 856–860 [DOI] [PubMed] [Google Scholar]
- Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, Yates JR, Lefkowitz RJ (2007) Functional specialization of β-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci USA 104: 12011–12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Fig S1