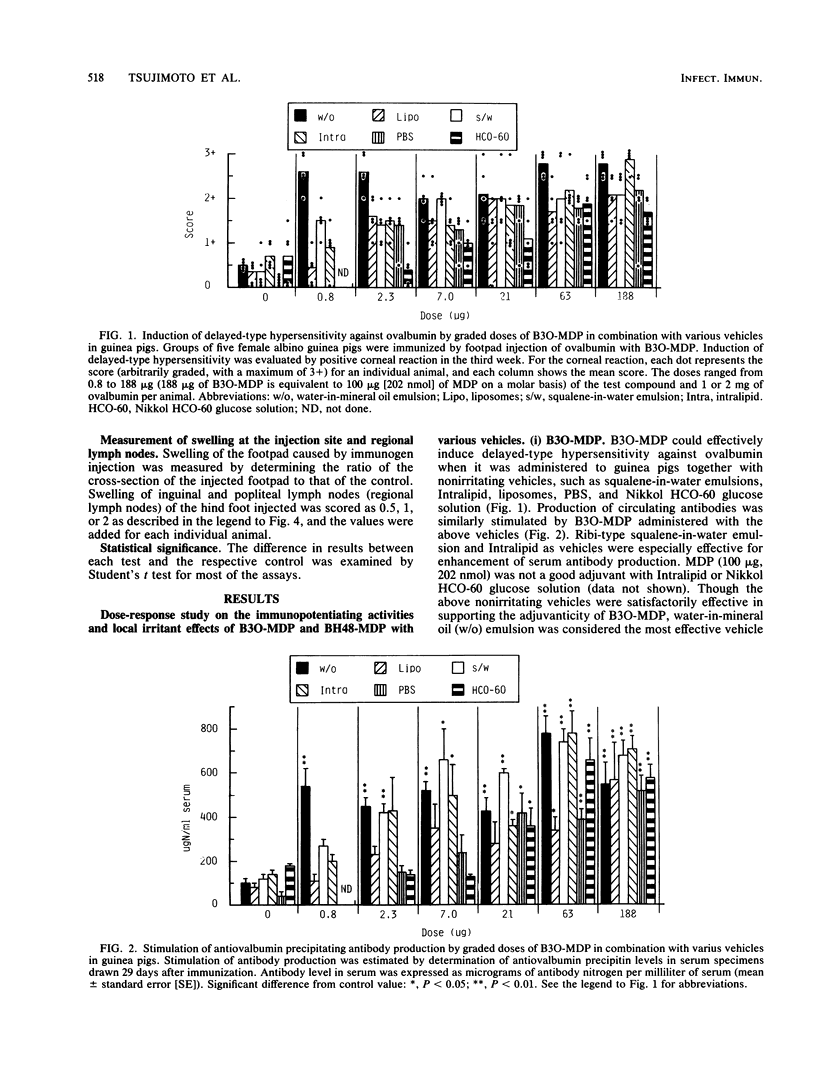
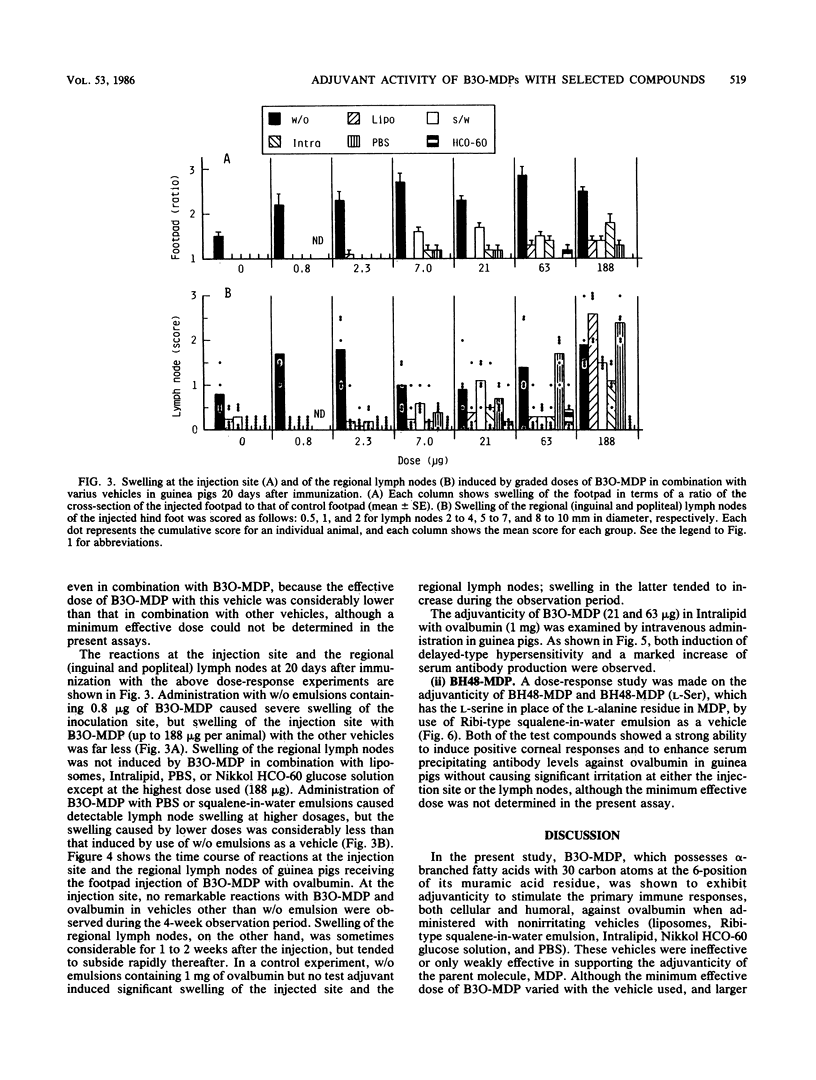
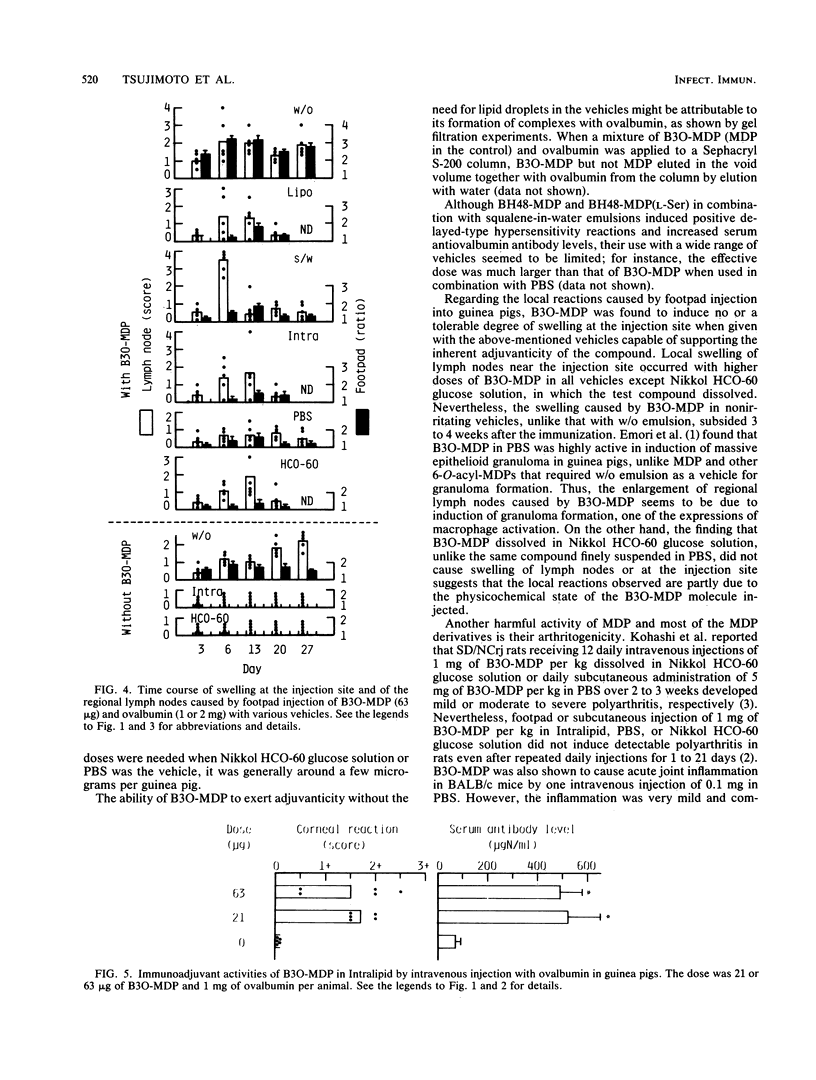
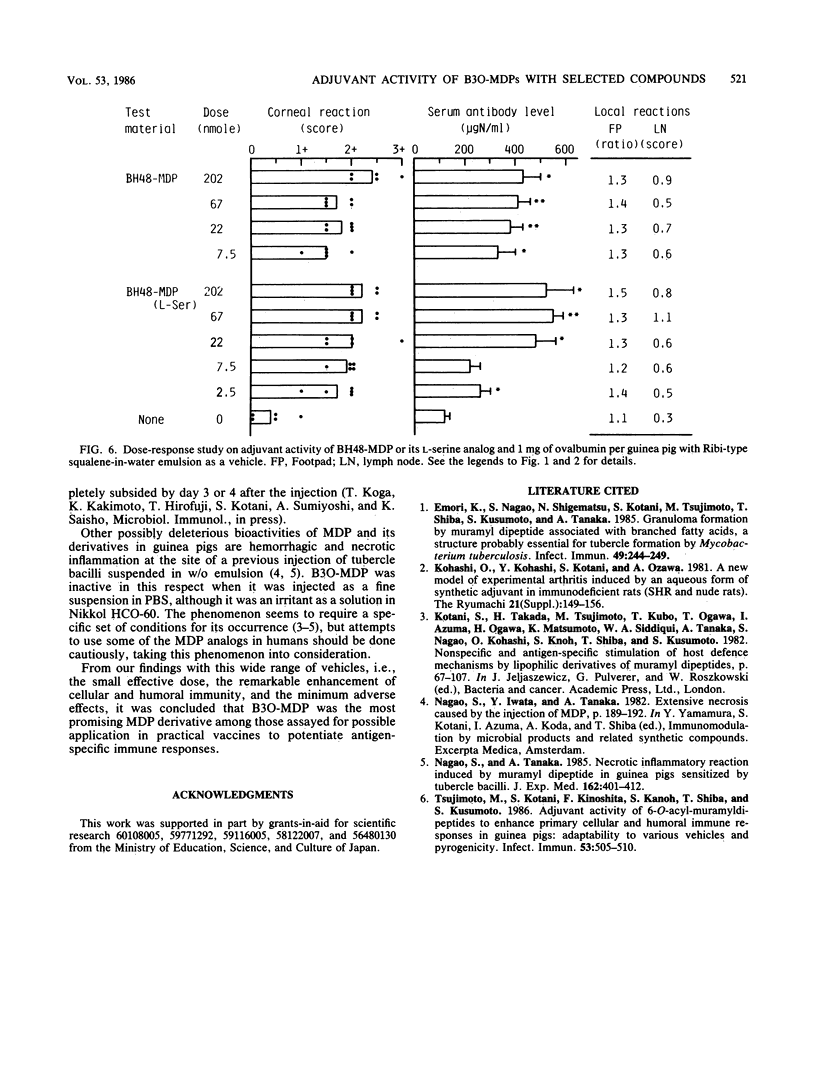
Abstract
6-O-(2-Tetradecylhexadecanoyl)-N-acetylmuramyl-L-alanyl-D-isoglutamine (B3O-MDP) and 6-O-(3-hydroxy-2-docosylhexacosanoyl)-MDP (BH48-MDP) were examined for immunopotentiating activity in stimulating the primary immune responses of guinea pigs to ovalbumin and for adverse reactions at the injection site and in the regional lymph nodes when administered in combination with several nonirritating vehicles. B30-MDP was found to potently stimulate both humoral and cellular immune responses, the latter by induction of delayed-type hypersensitivity, irrespective of the administration vehicle examined (liposomes, a squalene in water emulsion, Intralipid, phosphate-buffered saline, and Nikkol HCO-60 glucose solution), although the minimum effective dose was dependent on the vehicle used. Reactions in the footpad receiving the injection were negligible. Noticeable local reaction consisted of swelling of the lymph nodes in the region of the injection, but the swelling was only noted with higher doses and subsided 3 to 4 weeks after immunization, unlike the persistent swelling caused by the administration of water-in-mineral oil emulsions with or without B30-MDP. BH48-MDP and its L-serine analog, BH48-MDP(L-Ser), which has L-serine in place of L-alanine in MDP, in combination with the squalene-in-water emulsion, also intensely stimulated both cellular and humoral antiovalbumin immune responses, but the effects of these compounds seemed to be influenced to a greater degree by the vehicles than by B30-MDP. Thus, B30-MDP was chosen from a series of a 6-O-acyl derivatives of MDP as the most promising candidate for possible application in practical vaccines.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Emori K., Nagao S., Shigematsu N., Kotani S., Tsujimoto M., Shiba T., Kusumoto S., Tanaka A. Granuloma formation by muramyl dipeptide associated with branched fatty acids, a structure probably essential for tubercle formation by Mycobacterium tuberculosis. Infect Immun. 1985 Jul;49(1):244–249. doi: 10.1128/iai.49.1.244-249.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rayos C., Lo R. Y., Shewen P. E., Beveridge T. J. Cloning of a serotype-specific antigen from Pasteurella haemolytica A1. Infect Immun. 1986 Sep;53(3):505–510. doi: 10.1128/iai.53.3.505-510.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohashi O., Kohashi Y., Kotani S., Osawa A. A new model of experimental arthritis induced by an aqueous form of synthetic adjuvant in immunodeficient rate (SHR and nude rats). Ryumachi. 1981;21 (Suppl):149–156. [PubMed] [Google Scholar]
- Nagao S., Tanaka A. Necrotic inflammatory reaction induced by muramyl dipeptide in guinea pigs sensitized by tubercle bacilli. J Exp Med. 1985 Aug 1;162(2):401–412. doi: 10.1084/jem.162.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]



