Abstract
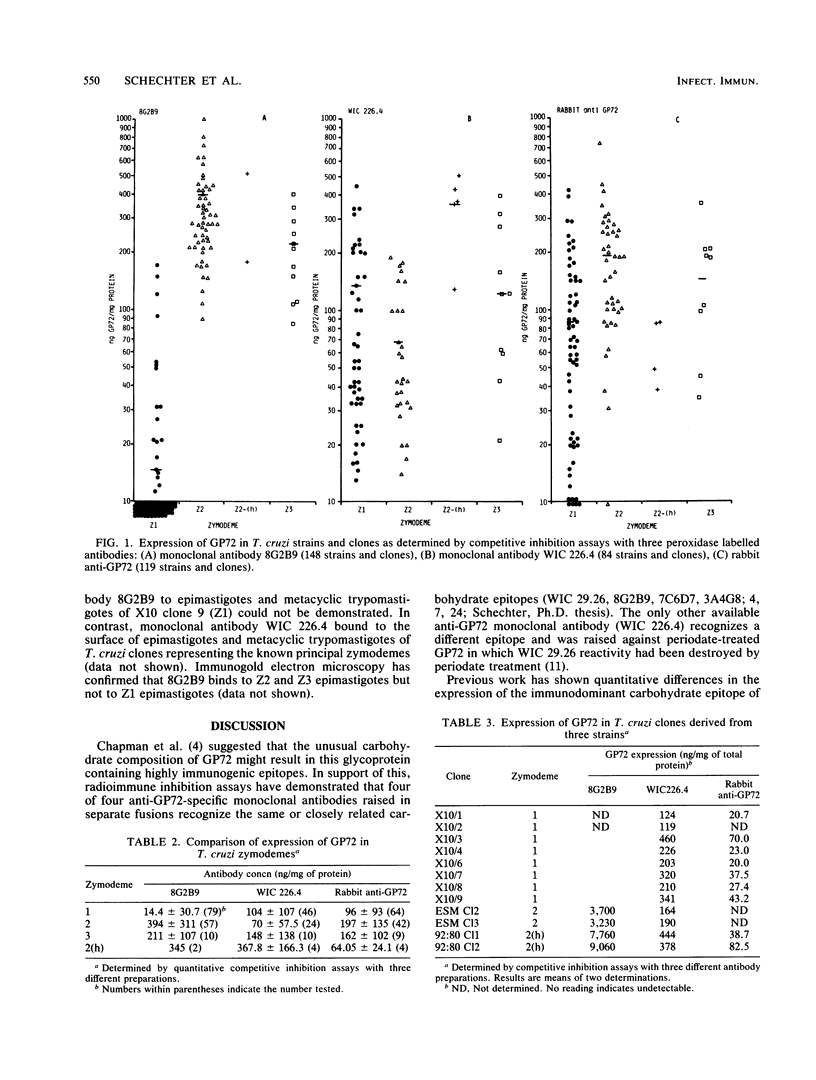
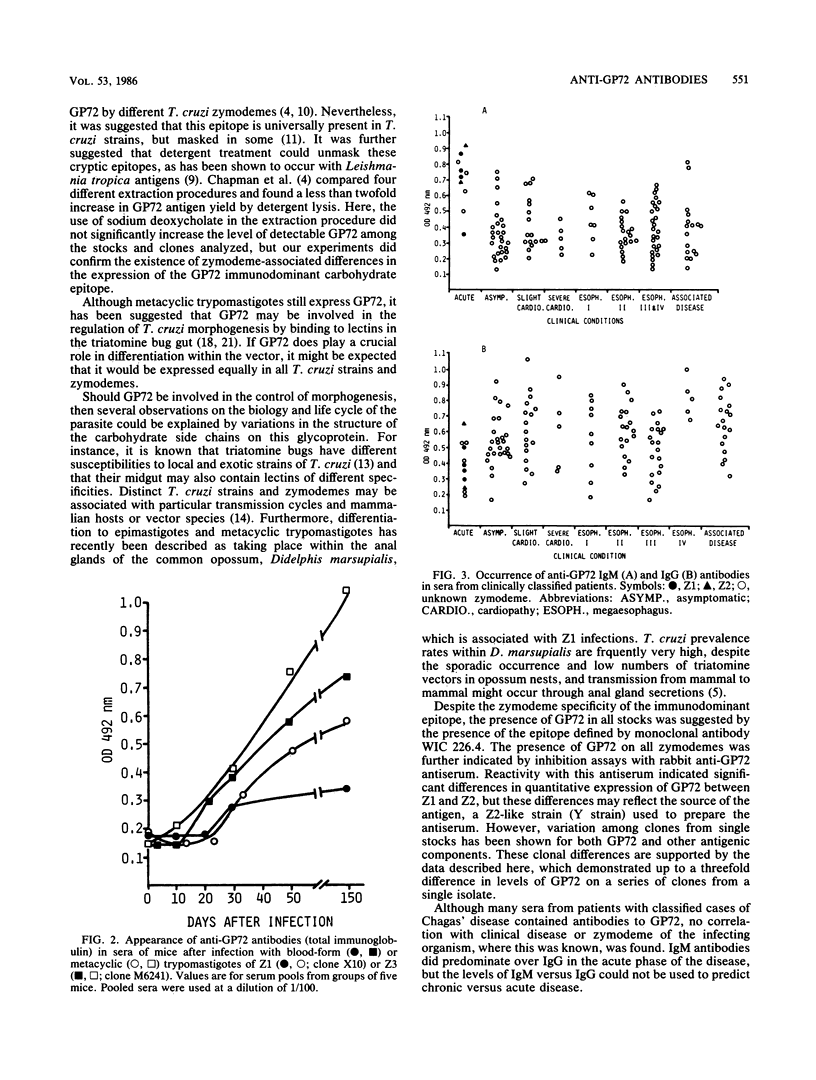
Three competitive inhibition enzyme-linked immunosorbent assays were developed to examine the expression of the 72-kilodalton glycoprotein (GP72) and of a GP72 carbohydrate epitope in Trypanosoma cruzi strains and clones. A total of 148 strains and clones of known isozyme phenotype (principal zymodeme, Z) were tested. With monoclonal antibody 8G2B9 the enzyme-linked immunosorbent assay confirmed that the majority of Z1 strains and clones derived from them had undetectable levels of the carbohydrate epitope identified by antibody 8G2B9. This epitope was, however, readily detectable in all Z2, Z2(h), and Z3 strains and clones (P less than 0.001; 148 strains and clones tested). Zymodeme-associated differences in GP72 expression were not apparent from the enzyme-linked immunosorbent assay with monoclonal antibody WIC 226.4 (raised against periodate-treated GP72) or from that with rabbit anti-GP72 antiserum (84 or 119 strains and clones tested, respectively). Mice infected with culture-form metacyclic trypomastigotes of Z1, Z29, and Z3 or with blood-form trypomastigotes of Z1 and Z3 developed antibodies to affinity-purified GP72, showing that at least some GP72 epitopes are neither zymodeme specific nor stage specific. A total of 128 serum samples from patients with acute or clinically classified chronic Chagas' disease were assayed for immunoglobulin G (IgG) or IgM anti-GP72 antibodies. During the acute phase anti-GP72 IgM antibodies were elevated, whereas anti-GP72 IgG antibodies were low. There were no significant differences in anti-GP72 antibody levels among chronic-phase patient groups. Anti-GP72 antibodies were detected irrespective of the geographical origin of patients and irrespective of whether acute-phase blood parasitemias were due to Z1 (four patients) or Z2 (two patients).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo F. G., Tighe L. Antigens of Trypanosoma cruzi: evidence that the 90-kd protective glycoprotein antigen is expressed in blood-form trypomastigotes and may not be functional in dead epimastigotes. J Parasitol. 1984 Feb;70(1):185–187. [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. Peroxidase labelled antibody and Fab conjugates with enhanced intracellular penetration. Immunochemistry. 1971 Dec;8(12):1175–1179. doi: 10.1016/0019-2791(71)90395-8. [DOI] [PubMed] [Google Scholar]
- Chapman M. D., Snary D., Miles M. A. Quantitative differences in the expression of a 72,000 molecular weight cell surface glycoprotein (GP72) in Trypanosoma cruzi zymodemes. J Immunol. 1984 Jun;132(6):3149–3153. [PubMed] [Google Scholar]
- Deane M. P., Lenzi H. L., Jansen A. Trypanosoma cruzi: vertebrate and invertebrate cycles in the same mammal host, the opossum Didelphis marsupialis. Mem Inst Oswaldo Cruz. 1984 Oct-Dec;79(4):513–515. doi: 10.1590/s0074-02761984000400021. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Allen A. K., Snary D. Studies on the structure of a phosphoglycoprotein from the parasitic protozoan Trypanosoma cruzi. Biochem J. 1983 Aug 1;213(2):313–319. doi: 10.1042/bj2130313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J. E., Schechter M., Chapman M. D., Miles M. A. Zymodeme and species specificities of monoclonal antibodies raised against Trypanosoma cruzi. Trans R Soc Trop Med Hyg. 1984;78(2):193–202. doi: 10.1016/0035-9203(84)90276-1. [DOI] [PubMed] [Google Scholar]
- Hoff R. A method for counting and concentrating living Trypanosoma cruzi in blood lysed with ammonium chloride. J Parasitol. 1974 Jun;60(3):527–528. [PubMed] [Google Scholar]
- Jaffe C. L., McMahon-Pratt D. Monoclonal antibodies specific for Leishmania tropica. I. Characterization of antigens associated with stage- and species-specific determinants. J Immunol. 1983 Oct;131(4):1987–1993. [PubMed] [Google Scholar]
- Kirchhoff L. V., Engel J. C., Dvorak J. A., Sher A. Strains and clones of Trypanosoma cruzi differ in their expression of a surface antigen identified by a monoclonal antibody. Mol Biochem Parasitol. 1984 Apr;11:81–89. doi: 10.1016/0166-6851(84)90056-2. [DOI] [PubMed] [Google Scholar]
- Kirchhoff L. V., Hieny S., Shiver G. M., Snary D., Sher A. Cryptic epitope explains the failure of a monoclonal antibody to bind to certain isolates of Trypanosoma cruzi. J Immunol. 1984 Nov;133(5):2731–2735. [PubMed] [Google Scholar]
- Luquetti A. O., Miles M. A., Rassi A., de Rezende J. M., de Souza A. A., Póvoa M. M., Rodrigues I. Trypanosoma cruzi: zymodemes associated with acute and chronic Chagas' disease in central Brazil. Trans R Soc Trop Med Hyg. 1986;80(3):462–470. doi: 10.1016/0035-9203(86)90347-0. [DOI] [PubMed] [Google Scholar]
- Miles M. A., Lanham S. M., de Souza A. A., Póvoa M. Further enzymic characters of Trypanosoma cruzi and their evaluation for strain identification. Trans R Soc Trop Med Hyg. 1980;74(2):221–237. doi: 10.1016/0035-9203(80)90251-5. [DOI] [PubMed] [Google Scholar]
- Miles M. A. The epidemiology of South American trypanosomiasis--biochemical and immunological approaches and their relevance to control. Trans R Soc Trop Med Hyg. 1983;77(1):5–23. doi: 10.1016/0035-9203(83)90004-4. [DOI] [PubMed] [Google Scholar]
- Nogueira N., Chaplan S., Tydings J. D., Unkeless J., Cohn Z. Trypanosoma cruzi. Surface antigens of blood and culture forms. J Exp Med. 1981 Mar 1;153(3):629–639. doi: 10.1084/jem.153.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco O., Afchain D., Dissous C., Rodriguez C., Ovlaque G., Lemesre J. L., Loyens M., Capron A. Different monoclonal antibodies against the component 5 specific for Trypanosoma cruzi. Am J Trop Med Hyg. 1984 Jul;33(4):560–568. doi: 10.4269/ajtmh.1984.33.560. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Andrade A. F., Ribeiro J. M. Lectins of distinct specificity in Rhodnius prolixus interact selectively with Trypanosoma cruzi. Science. 1981 Feb 6;211(4482):597–600. doi: 10.1126/science.7006082. [DOI] [PubMed] [Google Scholar]
- Póvoa M. M., de Souza A. A., Naiff R. D., Arias J. R., Naiff M. F., Biancardi C. B., Miles M. A. Chagas' disease in the Amazon basin IV. Host records of Trypanosoma cruzi zymodemes in the states of Amazonas and Rondonia, Brazil. Ann Trop Med Parasitol. 1984 Oct;78(5):479–487. [PubMed] [Google Scholar]
- Selden L. F., Baker J. R. Aseptic separation of cultivated trypomastigotes from epimastigotes of Trypanosoma (Schizotrypanum) dionisii, using DEAE cellulose. Trans R Soc Trop Med Hyg. 1980;74(3):406–407. doi: 10.1016/0035-9203(80)90114-5. [DOI] [PubMed] [Google Scholar]
- Sher A., Snary D. Specific inhibition of the morphogenesis of Trypanosoma cruzi by a monoclonal antibody. Nature. 1982 Dec 16;300(5893):639–640. doi: 10.1038/300639a0. [DOI] [PubMed] [Google Scholar]
- Snary D. Biochemistry of surface antigens of Trypanosoma cruzi. Br Med Bull. 1985 Apr;41(2):144–148. doi: 10.1093/oxfordjournals.bmb.a072041. [DOI] [PubMed] [Google Scholar]
- Snary D. Cell surface glycoproteins of Trypanosoma cruzi: protective immunity in mice and antibody levels in human chagasic sera. Trans R Soc Trop Med Hyg. 1983;77(1):126–129. doi: 10.1016/0035-9203(83)90037-8. [DOI] [PubMed] [Google Scholar]
- Snary D., Ferguson M. A., Scott M. T., Allen A. K. Cell surface antigens of Trypanosoma cruzi: use of monoclonal antibodies to identify and isolate an epimastigote specific glycoprotein. Mol Biochem Parasitol. 1981 Oct;3(6):343–356. doi: 10.1016/0166-6851(81)90035-9. [DOI] [PubMed] [Google Scholar]
- Tibayrenc M., Miles M. A. A genetic comparison between Brazilian and Bolivian zymodemes of Trypanosoma cruzi. Trans R Soc Trop Med Hyg. 1983;77(1):76–83. doi: 10.1016/0035-9203(83)90021-4. [DOI] [PubMed] [Google Scholar]


