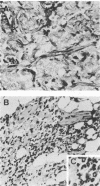
Abstract
Monoclonal antibodies directed to Mycobacterium bovis BCG (BCG) and to M. tuberculosis H37Rv (H37Rv) were used in conjunction with affinity chromatography to prepare a mycobacterial component which was designated BCG-a. A synthetic peptide antigen was prepared based on the amino acid sequence of BCG-a and was designated BCG-a-P. Significant immunological similarities were found between BCG-a-P and antigens in extracts of BCG and H37Rv but not between BCG-a-P and antigens of nontuberculous mycobacteria. An enzyme-linked immunosorbent assay detected antibodies to BCG-a-P in sera from rabbits that had been immunized with BCG and H37Rv sonicates. In Western blot analysis, antibodies to BCG-a-P reacted to 10,000-molecular-weight components of extracts of BCG and H37Rv. Delayed cutaneous hypersensitivity reactions to BCG-a-P were elicited in guinea pigs immunized with sonicates of BCG and H37Rv but were weak or nonexistent in unimmunized animals or in animals immunized with sonicates of nontuberculous mycobacteria. This study points out the feasibility of using monoclonal antibodies to prepare and characterize synthetic mycobacterial peptides with a potential for immunodiagnostic purposes.
Full text
PDF

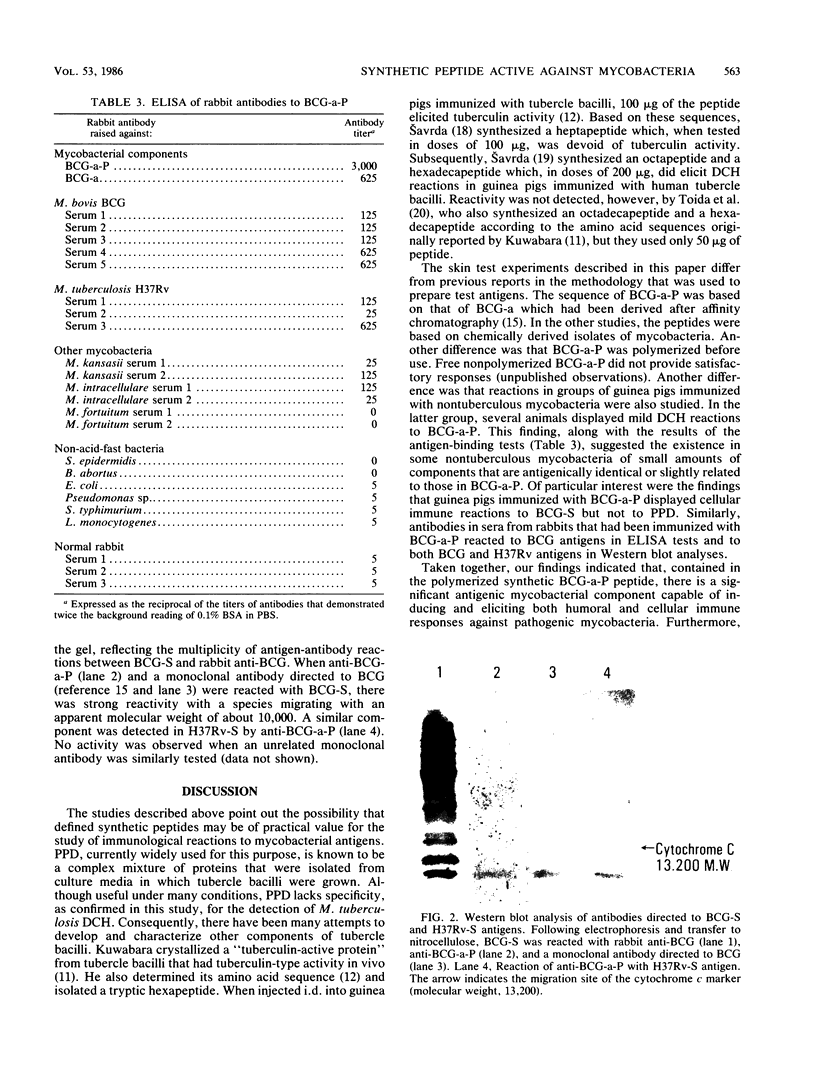

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker L. A., Sharpton T., Minden P., Campbell P. A. Adjuvant and mitogenic properties of a supernatant fraction of sonically treated Myobacterium bovis (BCG). Infect Immun. 1976 Jul;14(1):83–87. doi: 10.1128/iai.14.1.83-87.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittle J. L., Houghten R. A., Alexander H., Shinnick T. M., Sutcliffe J. G., Lerner R. A., Rowlands D. J., Brown F. Protection against foot-and-mouth disease by immunization with a chemically synthesized peptide predicted from the viral nucleotide sequence. Nature. 1982 Jul 1;298(5869):30–33. doi: 10.1038/298030a0. [DOI] [PubMed] [Google Scholar]
- Coates A. R., Hewitt J., Allen B. W., Ivanyi J., Mitchison D. A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981 Jul 25;2(8239):167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Janicki B. W. Mycobacterial antigens: a review of their isolation, chemistry, and immunological properties. Microbiol Rev. 1978 Mar;42(1):84–113. doi: 10.1128/mr.42.1.84-113.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis T. P., Buchanan T. M. Production and partial characterization of monoclonal antibodies to Mycobacterium leprae. Infect Immun. 1982 Jul;37(1):172–178. doi: 10.1128/iai.37.1.172-178.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B. Immunoreactive substances of mycobacteria. Am Rev Respir Dis. 1982 Mar;125(3 Pt 2):50–69. doi: 10.1164/arrd.1982.125.3P2.50. [DOI] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolk A. H., Ho M. L., Klatser P. R., Eggelte T. A., Kuijper S., de Jonge S., van Leeuwen J. Production and characterization of monoclonal antibodies to Mycobacterium tuberculosis, M. bovis (BCG) and M. leprae. Clin Exp Immunol. 1984 Dec;58(3):511–521. [PMC free article] [PubMed] [Google Scholar]
- Kuwabara S. Amino acid sequence of tuberculin-active protein from Mycobacterium tuberculosis. J Biol Chem. 1975 Apr 10;250(7):2563–2568. [PubMed] [Google Scholar]
- Kuwabara S. Purification and properties of tuberculin-active protein from Mycobacterium tuberculosis. J Biol Chem. 1975 Apr 10;250(7):2556–2562. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Minden P., Kelleher P. J., Freed J. H., Nielsen L. D., Brennan P. J., McPheron L., McClatchy J. K. Immunological evaluation of a component isolated from Mycobacterium bovis BCG with a monoclonal antibody to M. bovis BCG. Infect Immun. 1984 Nov;46(2):519–525. doi: 10.1128/iai.46.2.519-525.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden P., McClatchy J. K., Cooper R., Bardana E. J., Jr, Farr R. S. Shared antigens between Mycobacterium bovis (BCG) and other bacterial species. Science. 1972 Apr 7;176(4030):57–58. doi: 10.1126/science.176.4030.57. [DOI] [PubMed] [Google Scholar]
- Minden P., McClatchy J. K., Farr R. S. Shared antigens between heterologous bacterial species. Infect Immun. 1972 Oct;6(4):574–582. doi: 10.1128/iai.6.4.574-582.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savrda J. Synthesis and biological assays of a peptide from a tuberculin-active protein. Infect Immun. 1980 Dec;30(3):686–693. doi: 10.1128/iai.30.3.686-693.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savrda J. Synthesis and biological assays of peptides from a tuberculin-active protein. Infect Immun. 1983 Jun;40(3):1163–1169. doi: 10.1128/iai.40.3.1163-1169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toida I., Yamamoto S., Takuma S., Suzuki T., Hirata M. Lack of tuberculin activity of synthetic peptides. Infect Immun. 1985 Dec;50(3):614–619. doi: 10.1128/iai.50.3.614-619.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]