Abstract
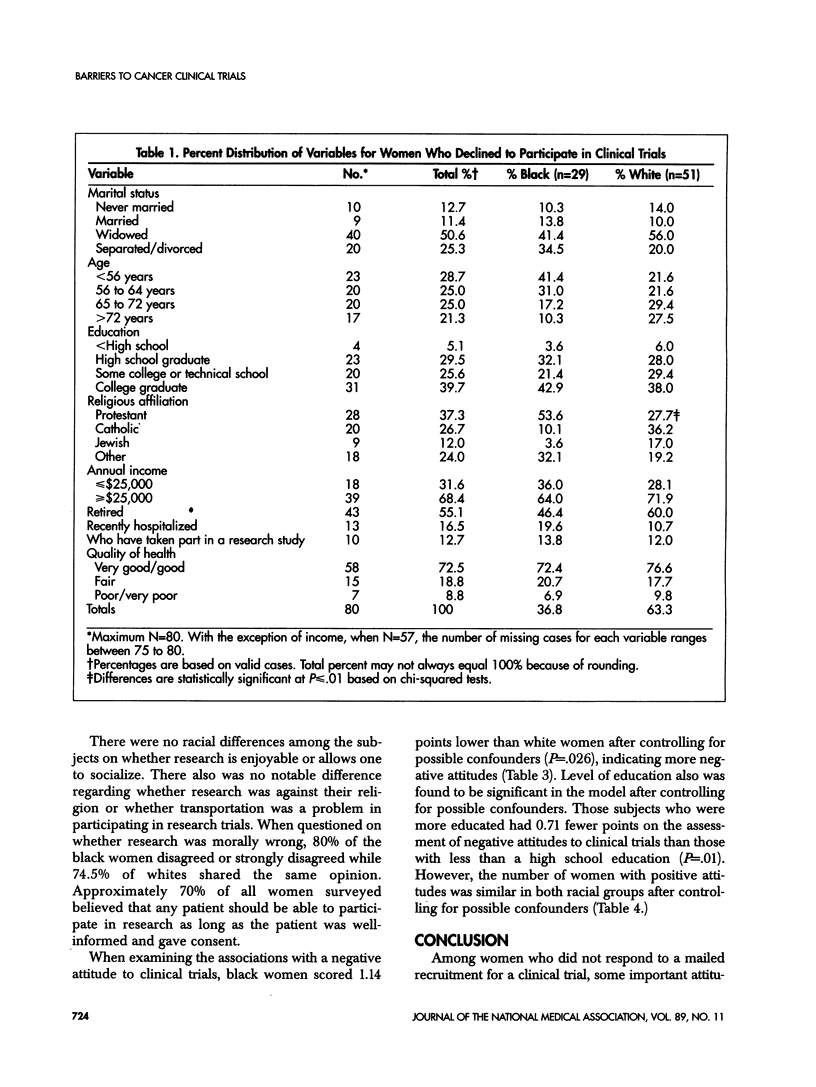
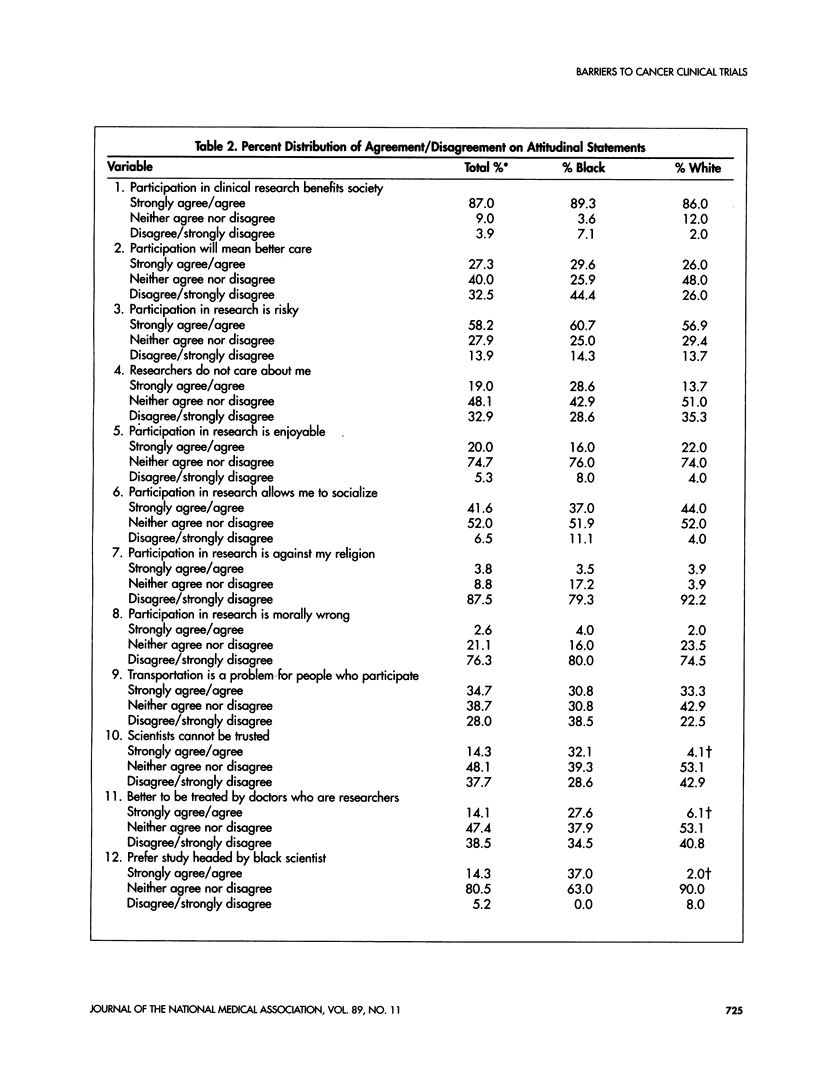
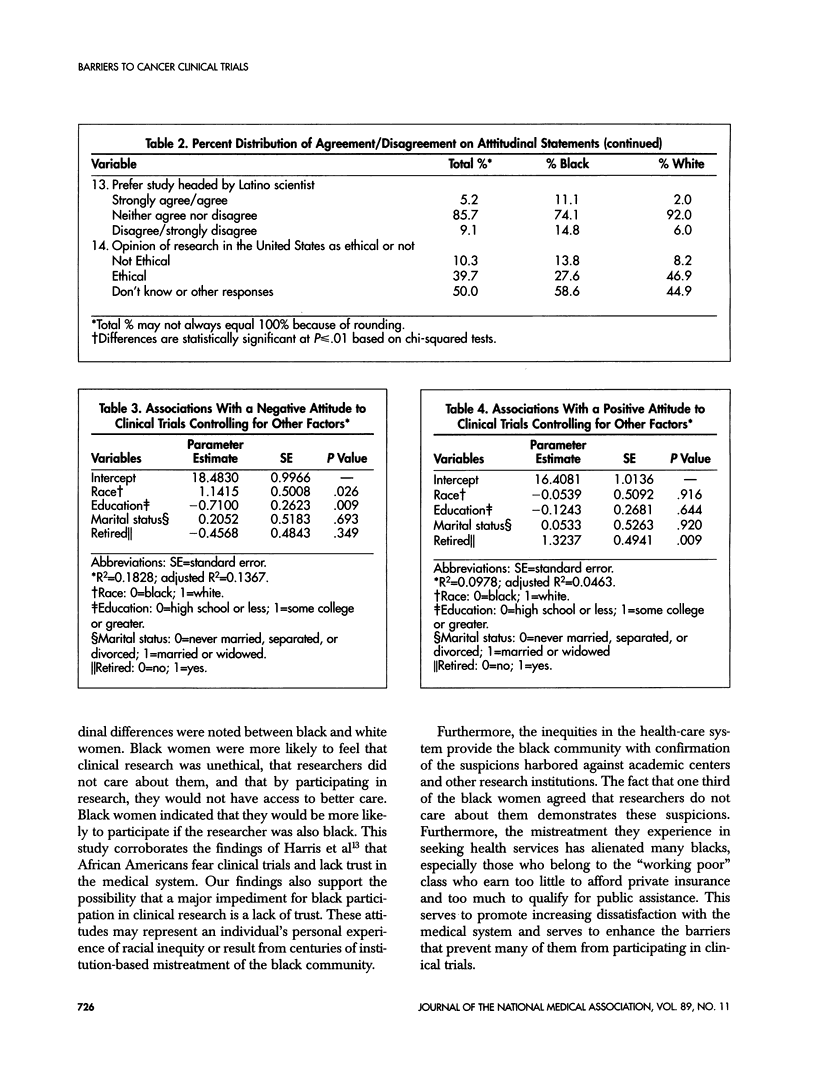
This study examines attitudes that may deter black women from participating in cancer research. Subjects were recruited from women who did not respond to the initial recruitment mailing for the Women's Health Initiative. Each subject was administered a 7- to 10-minute telephone survey. One third (29) of the 80 subjects were black. Fifty-six percent of black women and 71% of white women had positive attitudes toward cancer clinical trials. More than 80% of the women surveyed agreed that clinical research benefits society and increases medical knowledge. However, almost one third of the black women agreed that scientists cannot be trusted while only 4% of whites responded similarly. Additionally, 29% of black women agreed that researchers did not care about them compared with 14% of white women. Only 28% of black women felt that clinical research in the United States was ethical, and 37% had a preference to be treated by a black scientist compared with 2% of whites. Controlling for other covariates, black women had more negative altitudes overall to clinical trials than white women. These findings support the likelihood that barriers exist for the participation of blacks and other minorities in clinical research. These barriers may impact the involvement of black women in cancer clinical trials. Improving trust and creating a perception of a caring attitude from investigators are important to overcoming these barriers. The inclusion of more black scientists as leaders of cancer clinical trials also may help improve these participation rates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fingerhut L. A., MaKuc D. M. Mortality among minority populations in the United States. Am J Public Health. 1992 Aug;82(8):1168–1170. doi: 10.2105/ajph.82.8.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris Y., Gorelick P. B., Samuels P., Bempong I. Why African Americans may not be participating in clinical trials. J Natl Med Assoc. 1996 Oct;88(10):630–634. [PMC free article] [PubMed] [Google Scholar]
- Hunter C. P., Frelick R. W., Feldman A. R., Bavier A. R., Dunlap W. H., Ford L., Henson D., Macfarlane D., Smart C. R., Yancik R. Selection factors in clinical trials: results from the Community Clinical Oncology Program Physician's Patient Log. Cancer Treat Rep. 1987 Jun;71(6):559–565. [PubMed] [Google Scholar]
- Johnson M. S. Perspectives in medicine. Generalizability of homogenous samples in clinical trials. J Assoc Acad Minor Phys. 1990;1(2):31–33. [PubMed] [Google Scholar]
- Millon-Underwood S., Sanders E., Davis M. Determinants of participation in state-of-the-art cancer prevention, early detection/screening, and treatment trials among African-Americans. Cancer Nurs. 1993 Feb;16(1):25–33. [PubMed] [Google Scholar]
- Svensson C. K. Representation of American blacks in clinical trials of new drugs. JAMA. 1989 Jan 13;261(2):263–265. [PubMed] [Google Scholar]


