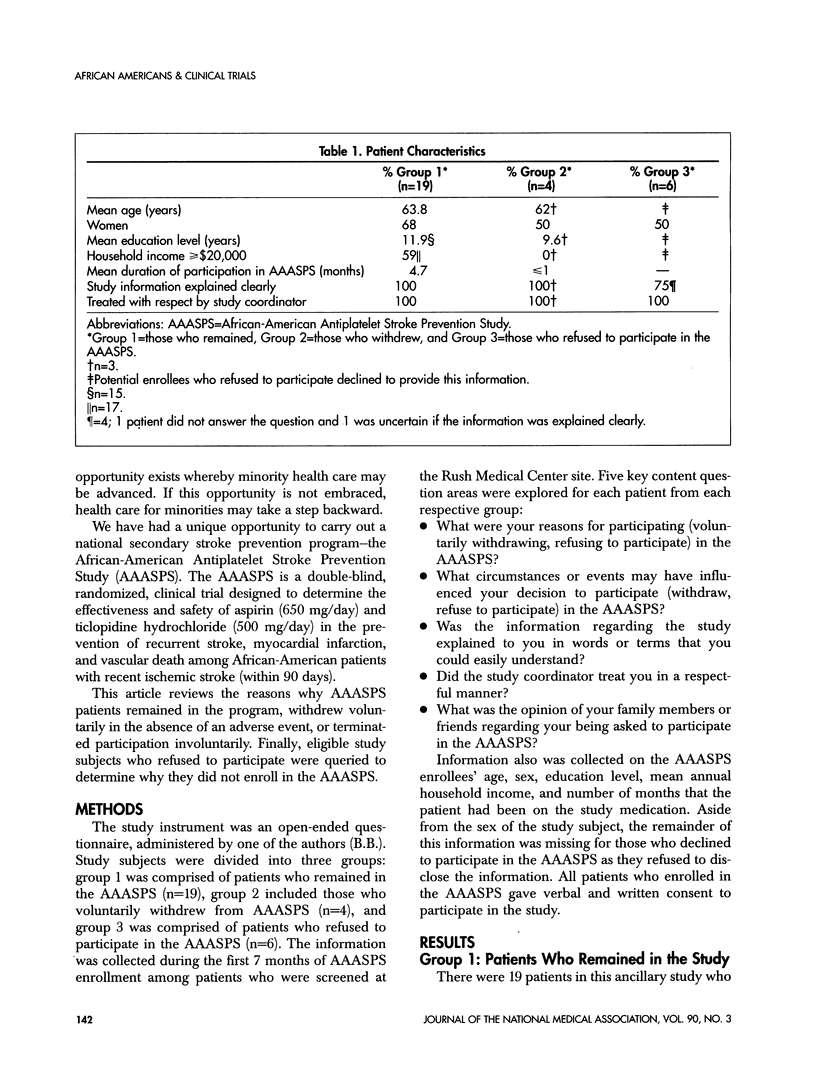
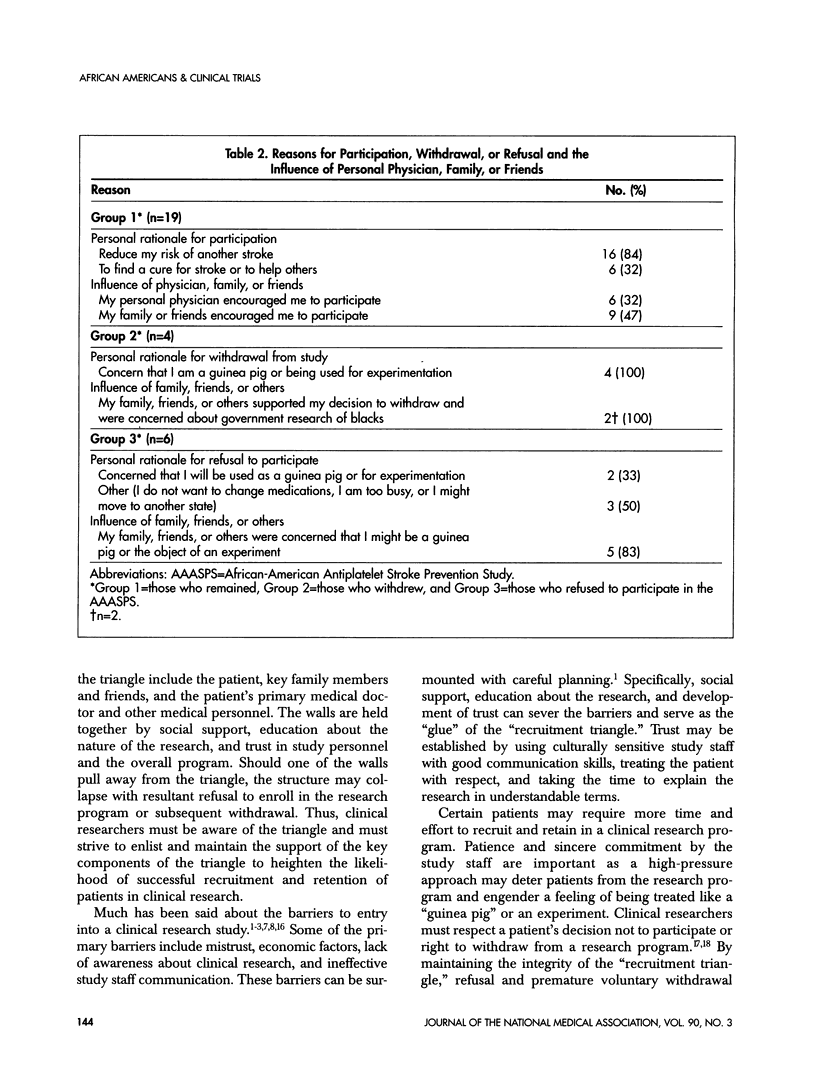
Abstract
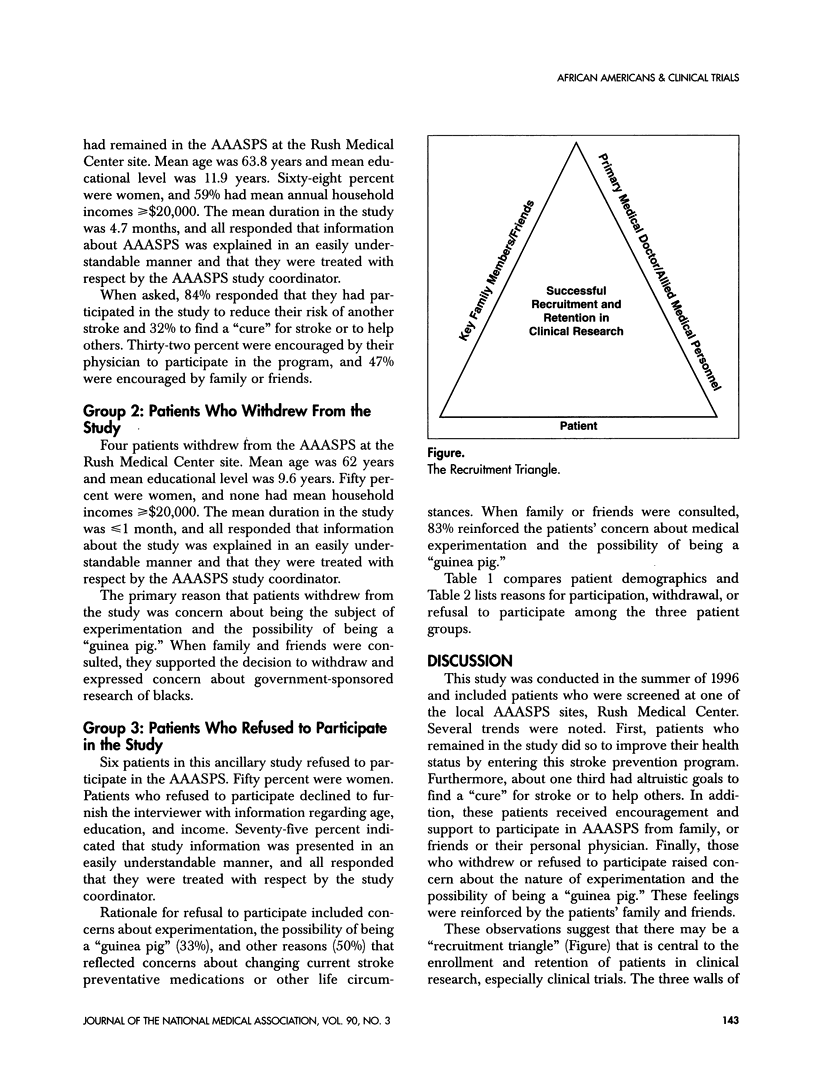
Recruitment and retention of study subjects are key to the success of a clinical trial. In the case of minority patients, this may be challenging as minority patients have been underserved by the medical health-care system. Furthermore, minority patients are more likely to experience barriers to entry into a clinical trial such as mistrust of the medical system, economic disadvantages, lack of awareness of study programs, and communication barriers. An open-ended questionnaire was used to determine reasons why subjects in the African-American Antiplatelet Stroke Prevention Study (AAASPS) remained in the study or voluntarily withdrew in the absence of an adverse event. Potential enrollees who refused to participate in the AAASPS also were queried. Enrollees who remained in the program consistently stated that they participated to reduce the risk of stroke recurrence and to help others by finding a "cure" for stroke. Those who withdrew or refused to participate consistently stated that they were afraid of being used as "guinea pigs." A "recruitment triangle" emerged that might predict a patient's likelihood of participation in a clinical trial. The sides of the triangle include the patient, key family members and friends, and the primary medical doctor and other medical personnel. The organizers of a clinical trial need to be aware of the "recruitment triangle" and establish strategies to heighten and maintain its integrity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. The dilemma for women of color in clinical trials. J Am Med Womens Assoc. 1994 Jul-Aug;49(4):105–109. [PubMed] [Google Scholar]
- Blendon R. J., Aiken L. H., Freeman H. E., Corey C. R. Access to medical care for black and white Americans. A matter of continuing concern. JAMA. 1989 Jan 13;261(2):278–281. [PubMed] [Google Scholar]
- Caplan A. L. Twenty years after. The legacy of the Tuskegee Syphilis Study. When evil intrudes. Hastings Cent Rep. 1992 Nov-Dec;22(6):29–32. [PubMed] [Google Scholar]
- Gavaghan H. Clinical trials face lack of minority group volunteers. Nature. 1995 Jan 19;373(6511):178–178. doi: 10.1038/373178a0. [DOI] [PubMed] [Google Scholar]
- Gorelick P. B., Richardson D., Hudson E., Perry C., Robinson D., Brown N., Harris Y. Establishing a community network for recruitment of African Americans into a clinical trial. The African-American antiplatelet stroke Prevention Study (AAASPS) experience. J Natl Med Assoc. 1996 Nov;88(11):701–704. [PMC free article] [PubMed] [Google Scholar]
- Harris Y., Gorelick P. B., Samuels P., Bempong I. Why African Americans may not be participating in clinical trials. J Natl Med Assoc. 1996 Oct;88(10):630–634. [PMC free article] [PubMed] [Google Scholar]
- Millon-Underwood S., Sanders E., Davis M. Determinants of participation in state-of-the-art cancer prevention, early detection/screening, and treatment trials among African-Americans. Cancer Nurs. 1993 Feb;16(1):25–33. [PubMed] [Google Scholar]
- Pinn V. W. The role of the NIH's Office of Research on Women's Health. Acad Med. 1994 Sep;69(9):698–702. doi: 10.1097/00001888-199409000-00003. [DOI] [PubMed] [Google Scholar]
- Roberson N. L. Clinical trial participation. Viewpoints from racial/ethnic groups. Cancer. 1994 Nov 1;74(9 Suppl):2687–2691. doi: 10.1002/1097-0142(19941101)74:9+<2687::aid-cncr2820741817>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Stoy D. B., Curtis R. C., Dameworth K. S., Dowdy A. A., Hegland J., Levin J. A., Sousoulas B. G. The successful recruitment of elderly black subjects in a clinical trial: the CRISP experience. Cholesterol Reduction in Seniors Program. J Natl Med Assoc. 1995 Apr;87(4):280–287. [PMC free article] [PubMed] [Google Scholar]
- Svensson C. K. Representation of American blacks in clinical trials of new drugs. JAMA. 1989 Jan 13;261(2):263–265. [PubMed] [Google Scholar]
- Swanson G. M., Ward A. J. Recruiting minorities into clinical trials: toward a participant-friendly system. J Natl Cancer Inst. 1995 Dec 6;87(23):1747–1759. doi: 10.1093/jnci/87.23.1747. [DOI] [PubMed] [Google Scholar]
- Thomas C. R., Jr, Pinto H. A., Roach M., 3rd, Vaughn C. B. Participation in clinical trials: is it state-of-the-art treatment for African Americans and other people of color? J Natl Med Assoc. 1994 Mar;86(3):177–182. [PMC free article] [PubMed] [Google Scholar]
- Veatch R. M. Consent, confidentiality, and research. N Engl J Med. 1997 Mar 20;336(12):869–870. doi: 10.1056/NEJM199703203361209. [DOI] [PubMed] [Google Scholar]



