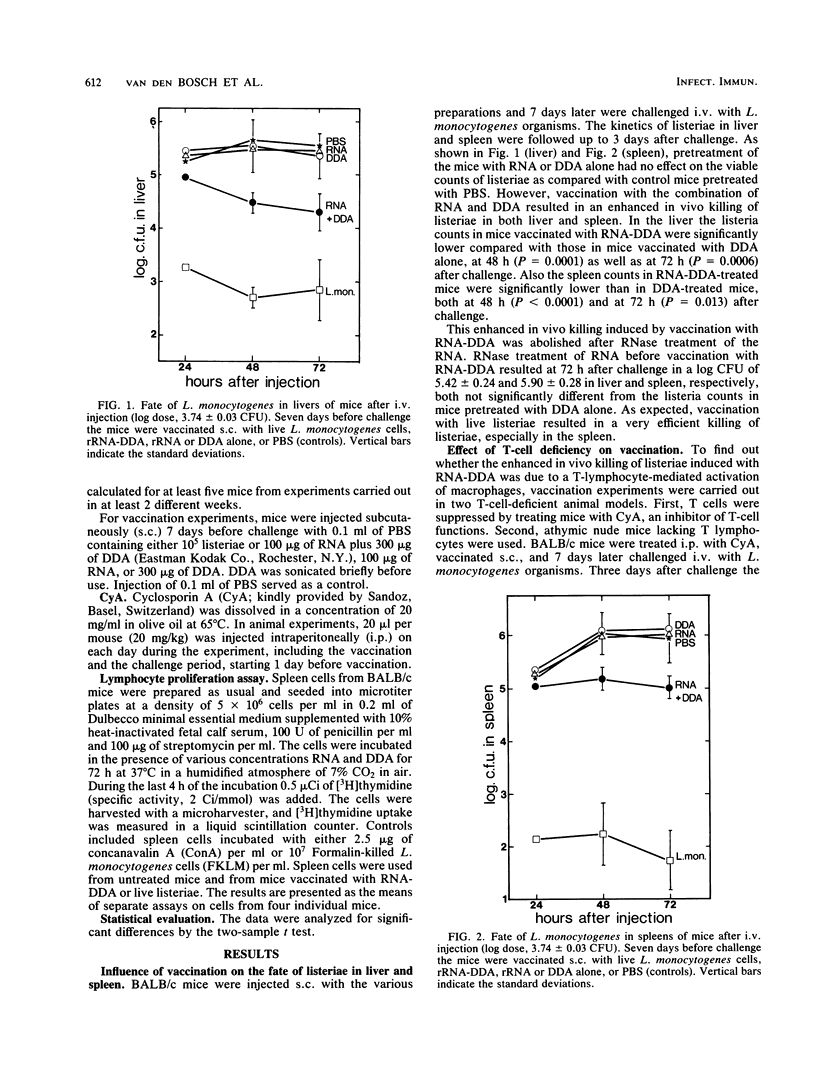
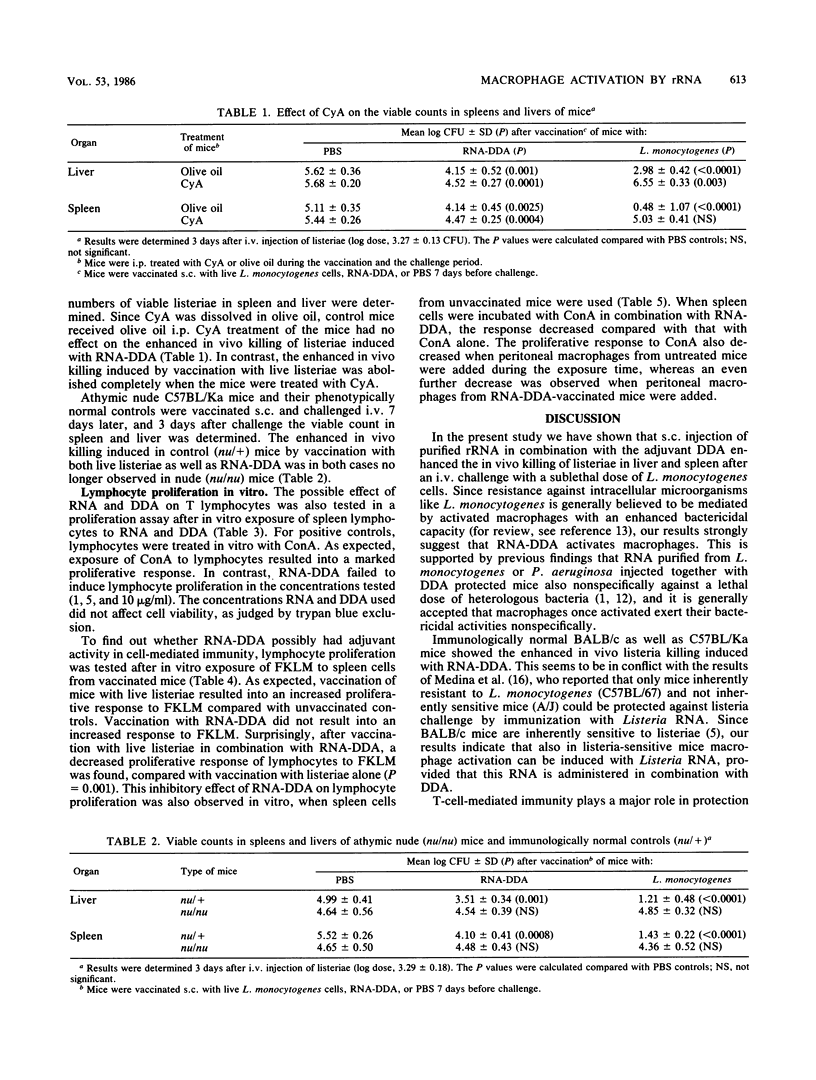
Abstract
Purified rRNA from Listeria monocytogenes or Pseudomonas aeruginosa injected in combination with dimethyldioctadecylammonium bromide (DDA), protects mice nonspecifically against a lethal challenge of various extra- and intracellular bacteria. In the present study vaccination of BALB/c as well as C57BL/Ka mice with listerial RNA-DDA resulted in activation of fixed-tissue macrophages, as measured by an enhanced in vivo L. monocytogenes killing in spleen and liver. Evidence was found that macrophage activation by vaccination with rRNA-DDA occurred by a T-cell-independent mechanism. Treatment of mice with cyclosporin A had no effect on the enhanced L. monocytogenes killing induced with RNA-DDA; in vitro exposure of RNA-DDA to spleen cell cultures did not give rise to any lymphocyte proliferation. No evidence could be found for a possible adjuvant activity for RNA-DDA in cellular responses; in fact, RNA-DDA had an inhibitory effect on lymphocyte proliferative responses to Listeria antigen and to concanavalin A.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonissen A. C., Lemmens P. J., Gonggrijp R., van den Bosch J. F., van Boven C. P. RNase-sensitive and RNase-insensitive protective components isolated from Listeria monocytogenes. Antonie Van Leeuwenhoek. 1985;51(2):227–240. doi: 10.1007/BF02310015. [DOI] [PubMed] [Google Scholar]
- Antonissen A. C., van Kessel K. P., van Dijk H., Willers J. M. Development of a simple passive haemagglutination-inhibition assay for Listeria monocytogenes lipoteichoic acid. J Immunol Methods. 1981;44(3):351–357. doi: 10.1016/0022-1759(81)90053-3. [DOI] [PubMed] [Google Scholar]
- Araujo F. G., Remington J. S. Protection against Toxoplasma gondii in mice immunized with Toxoplasma cell fractions, RNA and synthetic polyribonucleotides. Immunology. 1974 Oct;27(4):711–721. [PMC free article] [PubMed] [Google Scholar]
- Butler R. C., Friedman H., Specter S. C., Eisenstein T. K. Induction of immunoenhancing factors for murine splenocyte cultures by Salmonella typhimurium ribosome and ribonucleic acid extracts. Infect Immun. 1981 Jun;32(3):1123–1127. doi: 10.1128/iai.32.3.1123-1127.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F., Pavlov H., Waid C., York J. Resistance and susceptibility of mice to bacterial infection: course of listeriosis in resistant or susceptible mice. Infect Immun. 1978 Mar;19(3):763–770. doi: 10.1128/iai.19.3.763-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Emmerling P., Finger H., Hof H. Cell-mediated resistance to infection with Listeria monocytogenes in nude mice. Infect Immun. 1977 Feb;15(2):382–385. doi: 10.1128/iai.15.2.382-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y., Kagaya K., Miura H., Shinoda T., Natori K., Yamazaki S. Biological and biochemical characterization of macrophage activating factor (MAF) in murine lymphocytes: physiocochemical similarity of MAF to gamma interferon (IFN-gamma). Microbiol Immunol. 1984;28(6):691–702. doi: 10.1111/j.1348-0421.1984.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Gonggrijp R., Antonissen A. C., van den Bosch J. F., van Boven C. P. Ribonuclease-sensitive ribosomal vaccines. Antonie Van Leeuwenhoek. 1984;50(5-6):763–774. doi: 10.1007/BF02386239. [DOI] [PubMed] [Google Scholar]
- Gonggrijp R., Mullers W. J., Lemmens P. J., van Boven C. P. Ribonuclease-sensitive ribosomal vaccine of Pseudomonas aeruginosa. Infect Immun. 1980 Jan;27(1):204–210. doi: 10.1128/iai.27.1.204-210.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonggrijp R., Mullers W. J., van Boven C. P. Serotype-nonspecific protection induced by ribonucleic acid isolated from the ribosomal vaccine of Pseudomonas aeruginosa. Infect Immun. 1981 Jul;33(1):178–185. doi: 10.1128/iai.33.1.178-185.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. T lymphocyte-macrophage interactions in cellular antibacterial immunity. Immunobiology. 1982 Apr;161(3-4):361–368. doi: 10.1016/S0171-2985(82)80093-4. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Kalman V. K., Klimpel G. R. Cyclosporin A inhibits the production of gamma interferon (IFN gamma), but does not inhibit production of virus-induced IFN alpha/beta. Cell Immunol. 1983 May;78(1):122–129. doi: 10.1016/0008-8749(83)90265-4. [DOI] [PubMed] [Google Scholar]
- Medina S., Vas S. I., Robson H. G. Effect of nonspecific stimulation on the defense mechanisms of inbred mice. J Immunol. 1975 Jun;114(6):1720–1725. [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Protective ability of Salmonella ribosomal protein and RNA in inbred mice. Infect Immun. 1978 Jul;21(1):286–291. doi: 10.1128/iai.21.1.286-291.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newborg M. F., North R. J. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [PubMed] [Google Scholar]
- Nickol A. D., Bonventre P. F. Anomalous high native resistance to athymic mice to bacterial pathogens. Infect Immun. 1977 Dec;18(3):636–645. doi: 10.1128/iai.18.3.636-645.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reem G. H., Cook L. A., Vilcek J. Gamma interferon synthesis by human thymocytes and T lymphocytes inhibited by cyclosporin A. Science. 1983 Jul 1;221(4605):63–65. doi: 10.1126/science.6407112. [DOI] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Davis C. E. Models of T cell deficiency in listeriosis: the effects of cortisone and cyclosporin A on normal and nude BALB/c mice. J Immunol. 1983 Jul;131(1):450–453. [PubMed] [Google Scholar]
- Svedersky L. P., Benton C. V., Berger W. H., Rinderknecht E., Harkins R. N., Palladino M. A. Biological and antigenic similarities of murine interferon-gamma and macrophage-activating factor. J Exp Med. 1984 Mar 1;159(3):812–827. doi: 10.1084/jem.159.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. W., Moon D. K., Nelson D. S. Suppression of delayed-type hypersensitivity reactions and lymphokine production by cyclosporin A in the mouse. Clin Exp Immunol. 1983 Jun;52(3):599–606. [PMC free article] [PubMed] [Google Scholar]
- Wiesinger D., Borel J. F. Studies on the mechanism of action of cyclosporin A. Immunobiology. 1980 Jan;156(4-5):454–463. doi: 10.1016/S0171-2985(80)80078-7. [DOI] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. IMMUNOGENIC ACTIVITY OF A RIBOSOMAL FRACTION OBTAINED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1965 May;89:1291–1298. doi: 10.1128/jb.89.5.1291-1298.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Effect of trypsin and ribonuclease on the immunogenic activity of ribosomes and ribonucleic acid isolated from Mycobacterium tuberculosis. J Bacteriol. 1966 Jun;91(6):2146–2154. doi: 10.1128/jb.91.6.2146-2154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V. Macrophage activation in mice lacking thymus-derived (T) cells. Experientia. 1975 May 15;31(5):591–593. doi: 10.1007/BF01932477. [DOI] [PubMed] [Google Scholar]


