Abstract
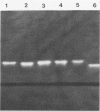
Superoxide dismutase (EC 1.15.1.1) (SOD) activity has been detected in crude cell extracts of representative strains of the Mycobacterium avium, M. intracellulare, and M. scrofulaceum (MAIS) group. Polyacrylamide gel electrophoresis demonstrated a single SOD activity band for each of the MAIS strains, though there were differences in mobility. All M. avium and M. intracellulare and two of five M. scrofulaceum strains demonstrated a single activity band of identical mobility (Rf = 0.83), while the SOD activity band for the three remaining M. scrofulaceum strains migrated farther (Rf = 0.85). The differences in mobility correlated with differences in sensitivity to NaN3 and H2O2. The SOD activities of the majority of the MAIS strains which displayed the slower-migrating activity band were inhibited 22 to 81% after 15 min of exposure to 5 mM H2O2, suggesting that both iron and manganese may be present in a single enzyme. The SOD activities of the three M. scrofulaceum strains which had the faster-migrating activity band were inhibited 100% after only 5 min of exposure to 5 mM H2O2 and exhibited greater sensitivity to 5 and 10 mM NaN3, characteristics of an iron-containing SOD. A concentration of 1 mM KCN did not cause inhibition of enzyme activity in any of the MAIS strains tested. Extracellular SOD activity was detected in four of six MAIS strains and was shown to be identical in mobility to the SOD activity of the crude extracts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asada K., Yoshikawa K., Takahashi M., Maeda Y., Enmanji K. Superoxide dismutases from a blue-green alga, Plectonema boryanum. J Biol Chem. 1975 Apr 25;250(8):2801–2807. [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baess I. Deoxyribonucleic acid relationships between different serovars of Mycobacterium avium, Mycobacterium intracellulare and Mycobacterium scrofulaceum. Acta Pathol Microbiol Immunol Scand B. 1983 Jun;91(3):201–203. doi: 10.1111/j.1699-0463.1983.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Beaman B. L., Scates S. M., Moring S. E., Deem R., Misra H. P. Purification and properties of a unique superoxide dismutase from Nocardia asteroides. J Biol Chem. 1983 Jan 10;258(1):91–96. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Britton L., Malinowski D. P., Fridovich I. Superoxide dismutase and oxygen metabolism in Streptococcus faecalis and comparisons with other organisms. J Bacteriol. 1978 Apr;134(1):229–236. doi: 10.1128/jb.134.1.229-236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikata Y., Kusunose E., Ichihara K., Kusunose M. Purification of superoxide dismutases from Mycobacterium phlei. Osaka City Med J. 1975;21(2):127–136. [PubMed] [Google Scholar]
- Cooper W. J., Zika R. G. Photochemical formation of hydrogen peroxide in surface and ground waters exposed to sunlight. Science. 1983 May 13;220(4598):711–712. doi: 10.1126/science.220.4598.711. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., McCord J. M., Fridovich I. Preparation and assay of superoxide dismutases. Methods Enzymol. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- DiGuiseppi J., Fridovich I. Oxygen toxicity in Streptococcus sanguis. The relative importance of superoxide and hydroxyl radicals. J Biol Chem. 1982 Apr 25;257(8):4046–4051. [PubMed] [Google Scholar]
- Filice G. A., Beaman B. L., Krick J. A., Remington J. S. Effects of human neutrophils and monocytes on Nocardia asteroides: failure of killing despite occurrence of the oxidative metabolic burst. J Infect Dis. 1980 Sep;142(3):432–438. doi: 10.1093/infdis/142.3.432. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Pratt P. F. In vitro response of murine alveolar and peritoneal macrophages to Mycobacterium intracellulare. Am Rev Respir Dis. 1983 Dec;128(6):1044–1047. doi: 10.1164/arrd.1983.128.6.1044. [DOI] [PubMed] [Google Scholar]
- George K. L., Parker B. C., Gruft H., Falkinham J. O., 3rd Epidemiology of infection by nontuberculous mycobacteria. II. Growth and survival in natural waters. Am Rev Respir Dis. 1980 Jul;122(1):89–94. doi: 10.1164/arrd.1980.122.1.89. [DOI] [PubMed] [Google Scholar]
- Gorse G. J., Fairshter R. D., Friedly G., Dela Maza L., Greene G. R., Cesario T. C. Nontuberculous mycobacterial disease. Experience in a southern California hospital. Arch Intern Med. 1983 Feb;143(2):225–228. doi: 10.1001/archinte.143.2.225. [DOI] [PubMed] [Google Scholar]
- Haffner P. H., Coleman J. E. Cu (II)-carbon bonding in cyanide complexes of copper enzymes. 13C splitting of the Cu(II) electron spin resonance. J Biol Chem. 1973 Oct 10;248(19):6626–6629. [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: inactivation of the enzyme. Biochemistry. 1975 Dec 2;14(24):5294–5299. doi: 10.1021/bi00695a010. [DOI] [PubMed] [Google Scholar]
- Ichihara K., Kusunose E., Kusunose M., Mori T. Superoxide dismutase from Mycobacterium lepraemurium. J Biochem. 1977 May;81(5):1427–1433. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970 Nov 25;245(22):6176–6181. [PubMed] [Google Scholar]
- Kusunose E., Ichihara K., Noda Y., Kusunose M. Superoxide dismutase from Mycobacterium tuberculosis. J Biochem. 1976 Dec;80(6):1343–1352. doi: 10.1093/oxfordjournals.jbchem.a131407. [DOI] [PubMed] [Google Scholar]
- Kusunose M., Noda Y., Ichihara K., Kusunose E. Superoxide dismutase from Mycobacterium species, strain Takeo. Arch Microbiol. 1976 May 3;108(1):65–73. doi: 10.1007/BF00425094. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. Inhibition of superoxide dismutases by azide. Arch Biochem Biophys. 1978 Aug;189(2):317–322. doi: 10.1016/0003-9861(78)90218-7. [DOI] [PubMed] [Google Scholar]
- Privalle C. T., Gregory E. M. Superoxide dismutase and O2 lethality in Bacteroides fragilis. J Bacteriol. 1979 Apr;138(1):139–145. doi: 10.1128/jb.138.1.139-145.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer W. B., Birn K. J., Jenkins P. A., Marks J. Infection with the avian-Battey group of mycobacteria in England and Wales. Br Med J. 1969 May 17;2(5654):412–415. doi: 10.1136/bmj.2.5654.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens G. J., Bannister J. V., Bannister W. H., Flohé L., Günzler W. A., Kim S. M., Otting F. The primary structure of Cu-Zn superoxide dismutase from Photobacterium leiognathi: evidence for a separate evolution of Cu-Zn superoxide dismutase in bacteria. Hoppe Seylers Z Physiol Chem. 1983 Jun;364(6):675–690. doi: 10.1515/bchm2.1983.364.1.675. [DOI] [PubMed] [Google Scholar]
- Steinman H. M. Bacteriocuprein superoxide dismutases in pseudomonads. J Bacteriol. 1985 Jun;162(3):1255–1260. doi: 10.1128/jb.162.3.1255-1260.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance P. G., Keele B. B., Jr, Rajagopalan K. V. Superoxide dismutase from Streptococcus mutans. Isolation and characterization of two forms of the enzyme. J Biol Chem. 1972 Aug 10;247(15):4782–4786. [PubMed] [Google Scholar]
- Wheeler P. R., Gregory D. Superoxide dismutase, peroxidatic activity and catalase in Mycobacterium leprae purified from armadillo liver. J Gen Microbiol. 1980 Dec;121(2):457–464. doi: 10.1099/00221287-121-2-457. [DOI] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]
- Wong B., Edwards F. F., Kiehn T. E., Whimbey E., Donnelly H., Bernard E. M., Gold J. W., Armstrong D. Continuous high-grade mycobacterium avium-intracellulare bacteremia in patients with the acquired immune deficiency syndrome. Am J Med. 1985 Jan;78(1):35–40. doi: 10.1016/0002-9343(85)90458-9. [DOI] [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. Superoxide radicals and phagocytosis. Arch Biochem Biophys. 1974 Apr 2;161(2):395–401. doi: 10.1016/0003-9861(74)90320-8. [DOI] [PubMed] [Google Scholar]
- Zakowski P., Fligiel S., Berlin G. W., Johnson L., Jr Disseminated Mycobacterium avium-intracellulare infection in homosexual men dying of acquired immunodeficiency. JAMA. 1982 Dec 10;248(22):2980–2982. doi: 10.1001/jama.1982.03330220024029. [DOI] [PubMed] [Google Scholar]