Abstract
A murine monoclonal antibody (MAb) was prepared against Pseudomonas aeruginosa immunotype-1 (It-1) lipopolysaccharide (LPS). The MAb bound It-1 LPS in the enzyme-linked immunosorbent assay and in the immunodiffusion and immunoblotting assays, agglutinated and opsonized It-1 bacteria, and protected against challenge with live It-1 organisms in a murine burn infection model. All of these activities were immunotype specific. Correlation of the opsonic and protective properties of the MAb with its recognition site on the LPS O side chain confirmed that such immunotype-specific determinants are important targets for protective antibodies in Pseudomonas disease. The functional equivalence of this MAb and polyclonal antibodies from hyperimmune plasma underscores the therapeutic potential of single MAbs which recognize critical determinants in the LPS O side chain.
Full text
PDF

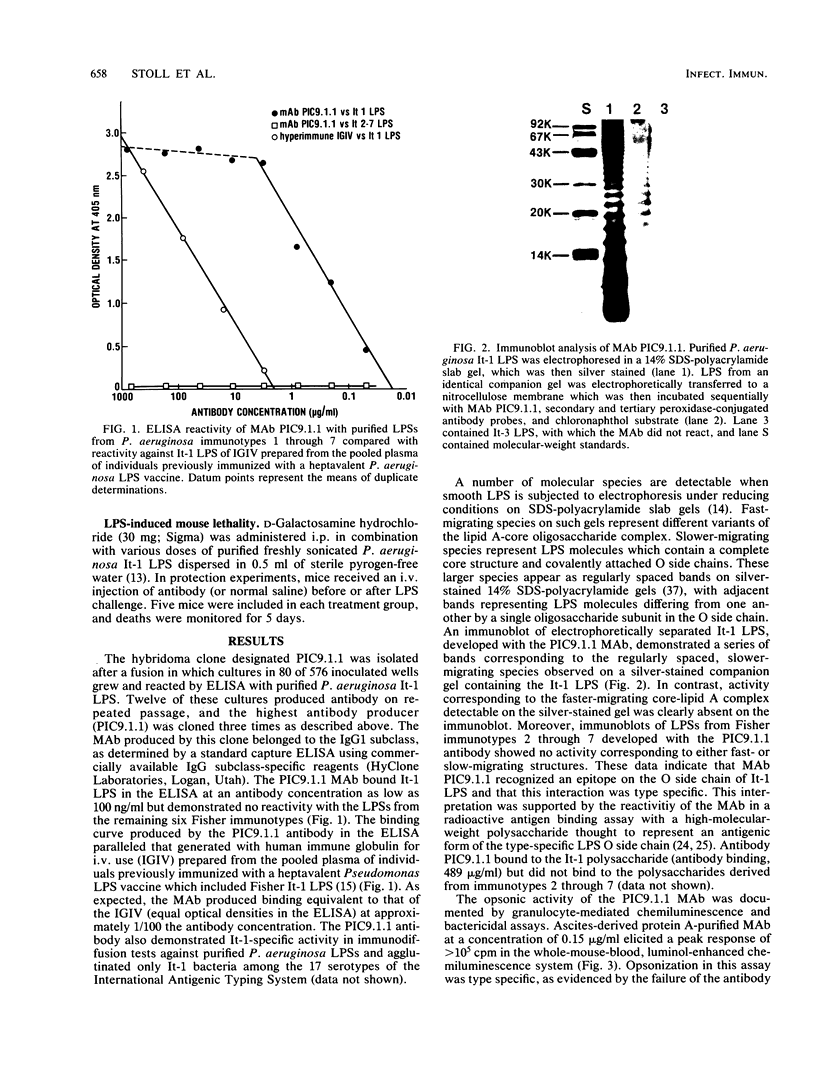
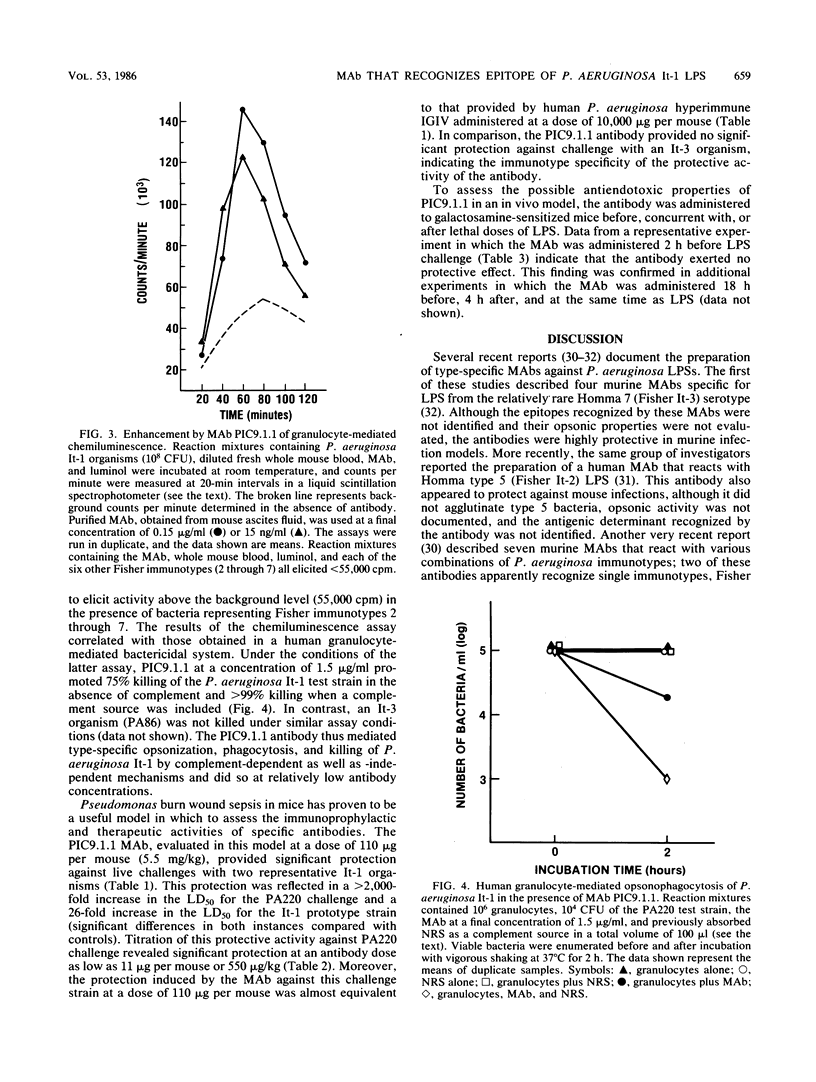
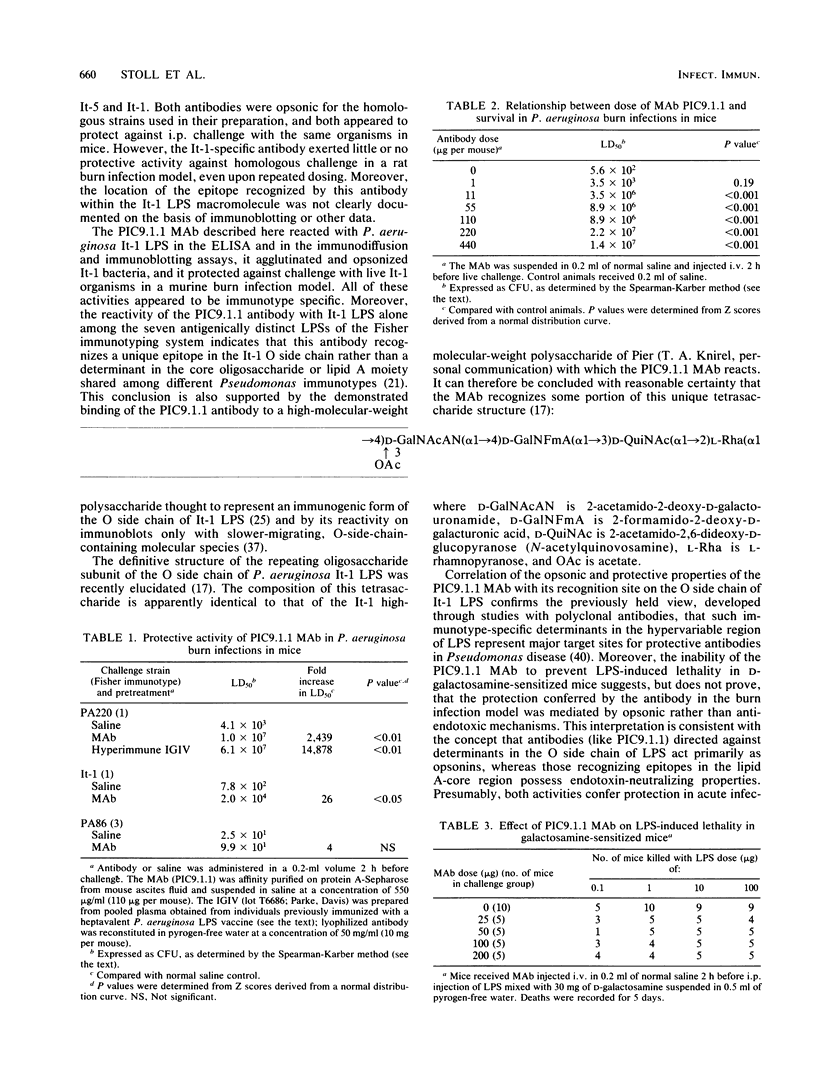


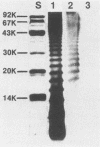
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brokopp C. D., Gomez-Lus R., Farmer J. J., 3rd Serological typing of Pseudomonas aeruginosa: use of commercial antisera and live antigens. J Clin Microbiol. 1977 Jun;5(6):640–649. doi: 10.1128/jcm.5.6.640-649.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Separation of leukocytes from blood and bone marrow. Introduction. Scand J Clin Lab Invest Suppl. 1968;97:7–7. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- Fischer G. W., Lowell G. H., Crumrine M. H., Bass J. W. Demonstration of opsonic activity and in vivo protection against group B streptococci type III by Streptococcus pneumoniae type 14 antisera. J Exp Med. 1978 Sep 1;148(3):776–786. doi: 10.1084/jem.148.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. W., Devlin H. B., Gnabasik F. J. New immunotype schema for Pseudomonas aeruginosa based on protective antigens. J Bacteriol. 1969 May;98(2):835–836. doi: 10.1128/jb.98.2.835-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Hanessian S., Regan W., Watson D., Haskell T. H. Isolation and characterization of antigenic components of a new heptavalent Pseudomonas vaccine. Nat New Biol. 1971 Feb 17;229(7):209–210. doi: 10.1038/newbio229209a0. [DOI] [PubMed] [Google Scholar]
- Knirel Y. A., Vinogradov E. V., Shashkov A. S., Dmitriev B. A., Kochetkov N. K., Stanislavsky E. S., Mashilova G. M. Somatic antigens of Pseudomonas aeruginosa. The structure of the O-specific polysaccharide chains of lipopolysaccharides of P. aeruginosa serogroup O4 (Lányi) and related serotype O6 (Habs) and immunotype 1 (Fisher). Eur J Biochem. 1985 Aug 1;150(3):541–550. doi: 10.1111/j.1432-1033.1985.tb09055.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Tanamoto K., Galanos C., McKenzie G. R., Brade H., Zähringer U., Rietschel E. T., Kusumoto S., Shiba T. Lipopolysaccharides: structural principles and biologic activities. Rev Infect Dis. 1984 Jul-Aug;6(4):428–431. doi: 10.1093/clinids/6.4.428. [DOI] [PubMed] [Google Scholar]
- Meadow P. M., Rowe P. S., Wells P. L. Characterization of polyagglutinating and surface antigens in Pseudomonas aeruginosa. J Gen Microbiol. 1984 Mar;130(3):631–644. doi: 10.1099/00221287-130-3-631. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Pollack M., Callahan L. T., 3rd, Iglewski B. H. Passive protection by antitoxin in experimental Pseudomonas aeruginosa burn infections. Infect Immun. 1977 Dec;18(3):596–602. doi: 10.1128/iai.18.3.596-602.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Markham R. B., Eardley D. Correlation of the biologic responses of C3H/HEJ mice to endotoxin with the chemical and structural properties of the lipopolysaccharides from Pseudomonas aeruginosa and Escherichia coli. J Immunol. 1981 Jul;127(1):184–191. [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Zolyomi S., Sadoff J. C. Isolation and characterization of a high-molecular-weight polysaccharide from the slime of Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):908–918. doi: 10.1128/iai.22.3.908-918.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Thomas D. M. Lipopolysaccharide and high-molecular-weight polysaccharide serotypes of Pseudomonas aeruginosa. J Infect Dis. 1982 Feb;145(2):217–223. doi: 10.1093/infdis/145.2.217. [DOI] [PubMed] [Google Scholar]
- Pollack M. Antibody activity against Pseudomonas aeruginosa in immune globulins prepared for intravenous use in humans. J Infect Dis. 1983 Jun;147(6):1090–1098. doi: 10.1093/infdis/147.6.1090. [DOI] [PubMed] [Google Scholar]
- Pollack M., Huang A. I., Prescott R. K., Young L. S., Hunter K. W., Cruess D. F., Tsai C. M. Enhanced survival in Pseudomonas aeruginosa septicemia associated with high levels of circulating antibody to Escherichia coli endotoxin core. J Clin Invest. 1983 Dec;72(6):1874–1881. doi: 10.1172/JCI111150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M. The virulence of Pseudomonas aeruginosa. Rev Infect Dis. 1984 Sep-Oct;6 (Suppl 3):S617–S626. doi: 10.1093/clinids/6.supplement_3.s617. [DOI] [PubMed] [Google Scholar]
- Pollack M., Young L. S. Protective activity of antibodies to exotoxin A and lipopolysaccharide at the onset of Pseudomonas aeruginosa septicemia in man. J Clin Invest. 1979 Feb;63(2):276–286. doi: 10.1172/JCI109300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J. C., Wright D. C., Futrovsky S., Sidberry H., Collins H., Kaufmann B. Characterization of mouse monoclonal antibodies directed against Pseudomonas aeruginosa lipopolysaccharides. Antibiot Chemother (1971) 1985;36:134–146. doi: 10.1159/000410478. [DOI] [PubMed] [Google Scholar]
- Sawada S., Suzuki M., Kawamura T., Fujinaga S., Masuho Y., Tomibe K. Protection against infection with Pseudomonas aeruginosa by passive transfer of monoclonal antibodies to lipopolysaccharides and outer membrane proteins. J Infect Dis. 1984 Oct;150(4):570–576. doi: 10.1093/infdis/150.4.570. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Stevens P., Young L. S. Quantitative granulocyte chemiluminescence in the rapid detection of impaired opsonization of Escherichia coli. Infect Immun. 1977 Jun;16(3):796–804. doi: 10.1128/iai.16.3.796-804.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieritz D. D., Holder I. A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis. 1975 Jun;131(6):688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G. Composition and structure of lipopolysaccharides from Pseudomonas aeruginosa. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S941–S949. doi: 10.1093/clinids/5.supplement_5.s941. [DOI] [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]
- Young L. S. Human immunity to Pseudomonas aeruginosa. II. Relationship between heat-stable opsonins and type-specific lipopolysaccharides. J Infect Dis. 1972 Sep;126(3):277–287. doi: 10.1093/infdis/126.3.277. [DOI] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]