Abstract
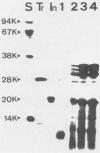
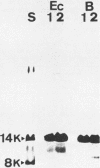
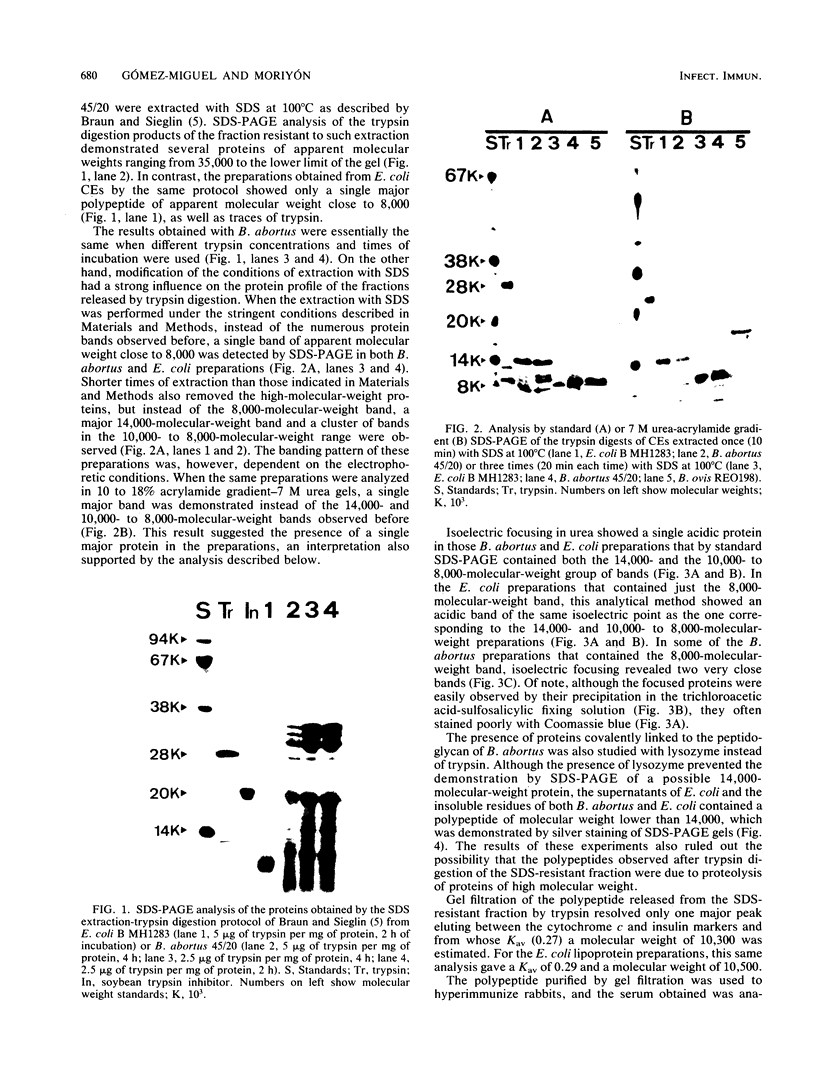
The sodium dodecyl sulfate (SDS) extraction-trypsin digestion protocol used by Braun and Sieglin (V. Braun and U. Sieglin, Eur. J. Biochem. 13:336-346, 1970) to show the peptidoglycan-linked lipoprotein of Escherichia coli was applied to both Brucella abortus and E. coli. Whereas a single polypeptide of 8,000 molecular weight was obtained from E. coli, several proteins of apparent molecular weight lower than 35,000 were demonstrated by SDS-polyacrylamide gel electrophoresis in B. abortus. These results did not change when the trypsin digestion conditions were modified. On the other hand, when the SDS extractions were performed under conditions more stringent than those used for other gram-negative bacteria, only a polypeptide fragment of apparent molecular weight of 8,000 was obtained from B. abortus. This polypeptide was similar to the trypsin fragment of the E. coli lipoprotein with respect to its behavior in SDS-polyacrylamide gels, isoelectric point in urea, molecular weight, and presence of both ester- and amide-linked fatty acids. Moreover, the amino acid analysis showed an overall similarity with respect to the amino acid composition of E. coli lipoprotein. A polypeptide of the same molecular weight, isoelectric point, and amino acid composition was also obtained from Brucella ovis by the same method. These results demonstrated that B. abortus and B. ovis cell envelopes contain a lipoprotein and strongly support the hypothesis that it is the only major protein covalently linked to the peptidoglycan.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgardner D., Deal C., Kaplan S. Protein composition of Rhodopseudomonas sphaeroides outer membrane. J Bacteriol. 1980 Jul;143(1):265–273. doi: 10.1128/jb.143.1.265-273.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Rotering H., Ohms J. P., Hagenmaier H. Conformational studies on murein-lipoprotein from the outer membrane of Escherichia coli. Eur J Biochem. 1976 Nov 15;70(2):601–610. doi: 10.1111/j.1432-1033.1976.tb11051.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Sieglin U. The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem. 1970 Apr;13(2):336–346. doi: 10.1111/j.1432-1033.1970.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Coloe P. J., Sinclair A. J., Slattery J. F., Burke D. Differentiation of Brucella ovis from Brucella abortus by gas-liquid chromatographic analysis of cellular fatty acids. J Clin Microbiol. 1984 Jun;19(6):896–898. doi: 10.1128/jcm.19.6.896-898.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dees S., Thanabalasundrum S., Moss C. W., Hollis D. G., Weaver R. E. Cellular fatty acid composition of group IVe, a nonsaccharolytic organism from clinical sources. J Clin Microbiol. 1980 Jun;11(6):664–668. doi: 10.1128/jcm.11.6.664-668.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Jones L. M., Wilson J. B. Antigenic relationship of Brucella ovis and Brucella melitensis. J Bacteriol. 1967 Apr;93(4):1262–1268. doi: 10.1128/jb.93.4.1262-1268.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubray G., Bézard G. Isolation of three Brucella abortus cell-wall antigens protective in murine experimental brucellosis. Ann Rech Vet. 1980;11(4):367–373. [PubMed] [Google Scholar]
- Dubray G., Charriaut C. Evidence of three major polypeptide species and two major polysaccharide species in the Brucella outer membrane. Ann Rech Vet. 1983;14(3):311–318. [PubMed] [Google Scholar]
- Dubray G., Plommet M. Structure et constituants des Brucella. Caractérisation des fractions et propriétés biologiques. Dev Biol Stand. 1976;31:68–91. [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O., Kim Y. B., Watson D. W. Biological activities of lipid A complexed with bovine-serum albumin. Eur J Biochem. 1972 Dec 4;31(2):230–233. doi: 10.1111/j.1432-1033.1972.tb02524.x. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Kroll H. P., Martin H. H. The covalent rigid-layer lipoprotein in cell walls of Proteus mirabilis. Eur J Biochem. 1978 Feb 1;83(1):227–233. doi: 10.1111/j.1432-1033.1978.tb12087.x. [DOI] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto F., Horigome T., Kanbayashi M., Yoshida K., Sugano H. An improved method for separation of low-molecular-weight polypeptides by electrophoresis in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1983 Feb 15;129(1):192–199. doi: 10.1016/0003-2697(83)90068-4. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Inouye M., Shaw J., Shen C. The assembly of a structural lipoprotein in the envelope of Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):8154–8159. [PubMed] [Google Scholar]
- Inoyye S., Takeishi K., Lee N., DeMartini M., Hirashima A., Inouye M. Lipoprotein from the outer membrane of Escherichia coli: purification, paracrystallization, and some properties of its free form. J Bacteriol. 1976 Jul;127(1):555–563. doi: 10.1128/jb.127.1.555-563.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. M., Diaz R., Berman D. T. Endotoxic activity of rough organisms of Brucella species. Infect Immun. 1976 Jun;13(6):1638–1641. doi: 10.1128/iai.13.6.1638-1641.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACAVE C., ROUX J. COMPOSITION CHIMIQUE DES PAROIS DE BRUCELLA. GLYCOSAMINOPEPTIDE DE BRUCELLA ABORTUS ET BRUCELLA MELITENSIS. C R Hebd Seances Acad Sci. 1965 Feb 1;260:1514–1517. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb V. L., Jones L. M., Schurig G. G., Berman D. T. Enzyme-linked immunosorbent assay for bovine immunoglobulin subclass-specific response to Brucella abortus lipopolysaccharides. Infect Immun. 1979 Oct;26(1):240–247. doi: 10.1128/iai.26.1.240-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Merril C. R., Switzer R. C., Van Keuren M. L. Trace polypeptides in cellular extracts and human body fluids detected by two-dimensional electrophoresis and a highly sensitive silver stain. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4335–4339. doi: 10.1073/pnas.76.9.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Isolation of characterization of a major outer membrane protein of Pseudomonas aeruginosa. Evidence for the occurrence of a lipoprotein. J Biochem. 1979 Jan;85(1):115–122. doi: 10.1093/oxfordjournals.jbchem.a132300. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J Biochem. 1978 Jul;84(1):179–191. doi: 10.1093/oxfordjournals.jbchem.a132106. [DOI] [PubMed] [Google Scholar]
- Moreno E., Pitt M. W., Jones L. M., Schurig G. G., Berman D. T. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979 May;138(2):361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyon I., Berman D. T. Effects of nonionic, ionic, and dipolar ionic detergents and EDTA on the Brucella cell envelope. J Bacteriol. 1982 Nov;152(2):822–828. doi: 10.1128/jb.152.2.822-828.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyon I., Berman D. T. Isolation, purification, and partial characterization of Brucella abortus matrix protein. Infect Immun. 1983 Jan;39(1):394–402. doi: 10.1128/iai.39.1.394-402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- SNYDER F., STEPHENS N. A simplified spectrophotometric determination of ester groups in lipids. Biochim Biophys Acta. 1959 Jul;34:244–245. doi: 10.1016/0006-3002(59)90255-0. [DOI] [PubMed] [Google Scholar]
- Santos J. M., Verstreate D. R., Perera V. Y., Winter A. J. Outer membrane proteins from rough strains of four Brucella species. Infect Immun. 1984 Oct;46(1):188–194. doi: 10.1128/iai.46.1.188-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag I., Schwarz H., Hirota Y., Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978 Oct;136(1):280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele O. W., Asselineau J., Lacave C. On the fatty acids of Brucella abortus and Brucella melitensis. Eur J Biochem. 1969 Jan;7(3):393–396. doi: 10.1111/j.1432-1033.1969.tb19621.x. [DOI] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]