Abstract
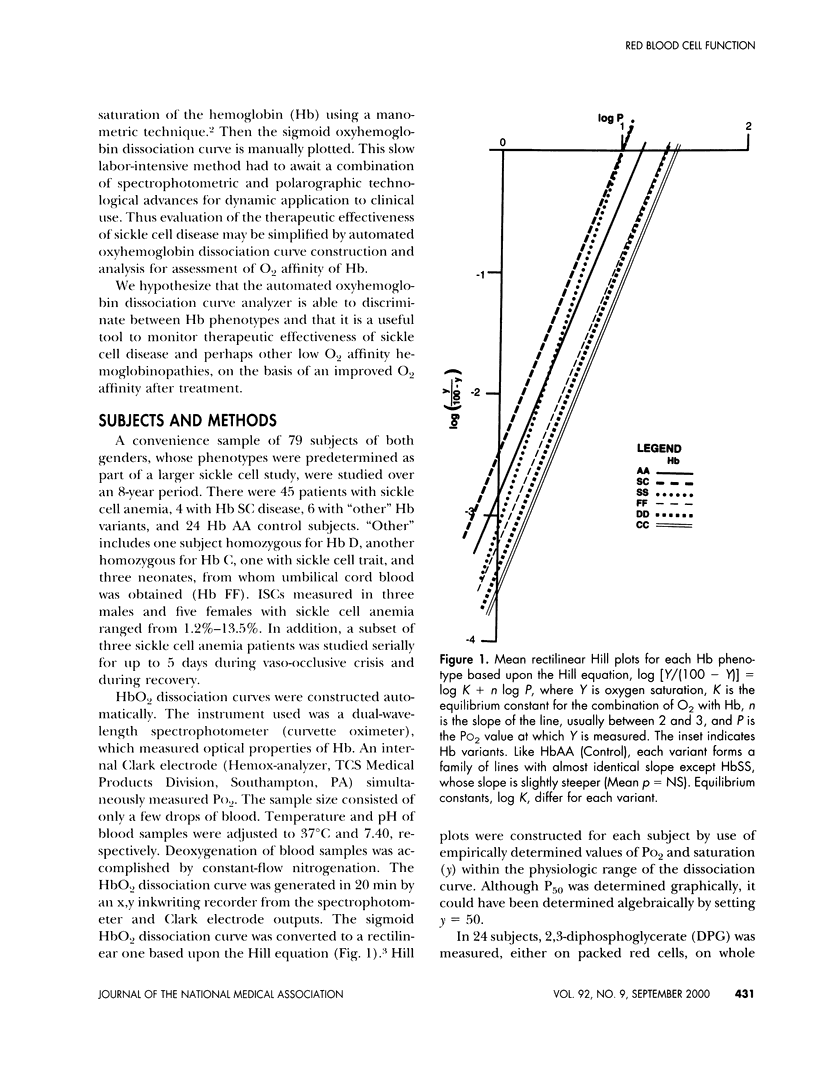
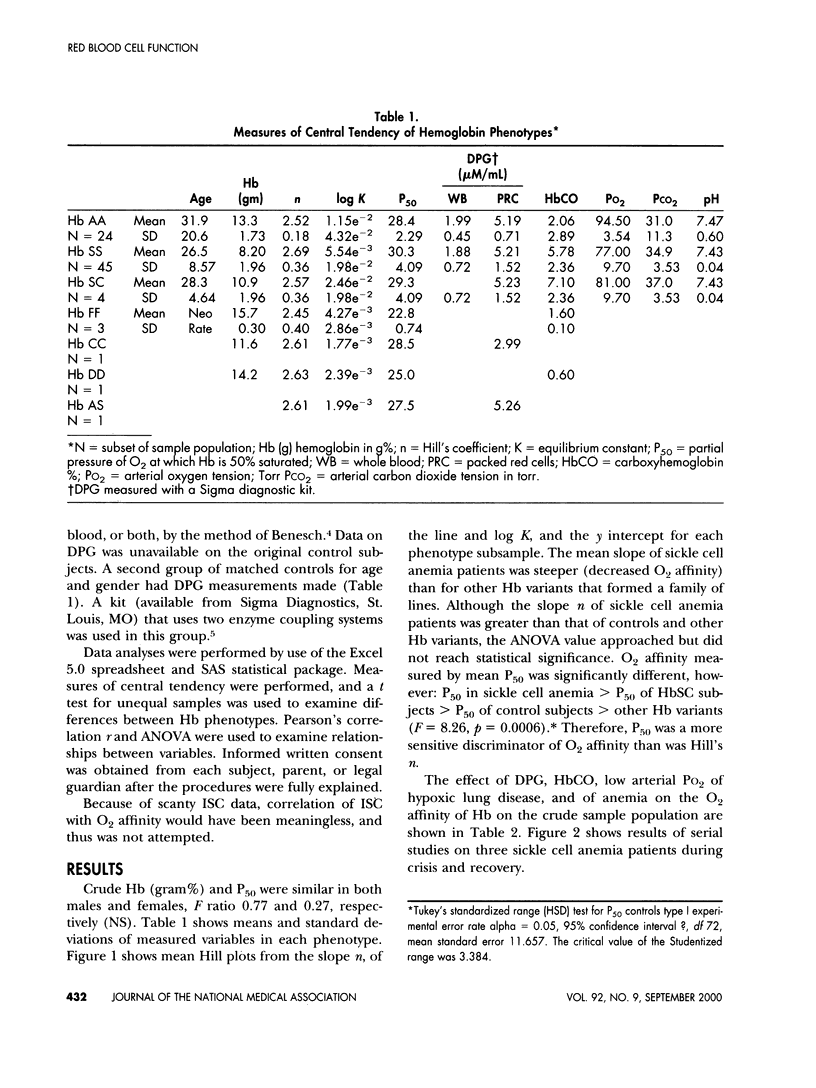
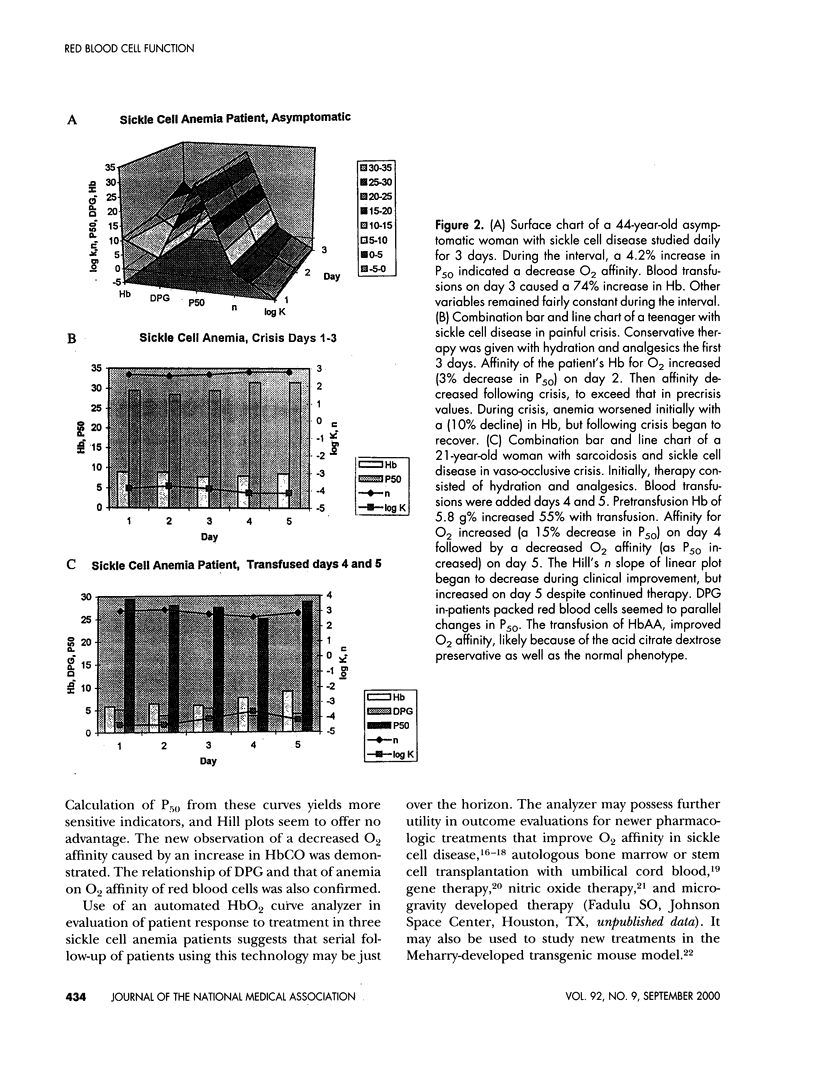
Oxyhemoglobin dissociation curves measure the most important function of red blood cells - the affinity for oxygen and its delivery to the tissues. This function may be deranged in sickle cell anemia and some other hemoglobinopathies. An automated oxyhemoglobin dissociation curve analyzer constructed dissociation curves in 55 patients with hemoglobinopathies and in 24 control subjects while maintaining constant temperature and pH. Sigmoid curves were converted to rectilinear ones using the Hill equation. Oxygen affinity of red cells was assessed by calculation of P50 (the PO2 at which hemoglobin is half saturated). Results revealed separation of oxyhemoglobin dissociation Hill plots according to phenotype but with wide variability. Mean oxygen affinity of fetal hemoglobin was greatest, whereas that of sickle hemoglobin was least. Other hemoglobins were intermediate. A positive correlation between decreased oxygen affinity and carboxyhemoglobin confirmed the decreased oxygen affinity of sickle hemoglobin and decreased oxygen affinity and increased diphosphoglycerate in red cells. Hill plots are less sensitive discriminators of oxygen affinity than traditional sigmoid dissociation curves and offer no particular advantage. Serial studies in a subset of three sickle cell anemia patients treated conservatively suggest automated oxyhemoglobin dissociation curves may be useful in assessment of effectiveness of newer therapies of sickle cell anemia after refinement of the method and studies of larger populations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benesch R., Benesch R. E., Yu C. I. Reciprocal binding of oxygen and diphosphoglycerate by human hemoglobin. Proc Natl Acad Sci U S A. 1968 Feb;59(2):526–532. doi: 10.1073/pnas.59.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookchin R. M., Lew V. L. Pathophysiology of sickle cell anemia. Hematol Oncol Clin North Am. 1996 Dec;10(6):1241–1253. doi: 10.1016/s0889-8588(05)70397-x. [DOI] [PubMed] [Google Scholar]
- Bromberg P. A., Jensen W. N. Blood oxygen dissociation curves in sickle cell disease. J Lab Clin Med. 1967 Sep;70(3):480–488. [PubMed] [Google Scholar]
- Gladwin M. T., Schechter A. N., Shelhamer J. H., Ognibene F. P. The acute chest syndrome in sickle cell disease. Possible role of nitric oxide in its pathophysiology and treatment. Am J Respir Crit Care Med. 1999 May;159(5 Pt 1):1368–1376. doi: 10.1164/ajrccm.159.5.9810094. [DOI] [PubMed] [Google Scholar]
- Hassan W., Beuzard Y., Rosa J. Inhibition of erythrocyte sickling by cystamine, a thiol reagent. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3288–3292. doi: 10.1073/pnas.73.9.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl R. J., Sherburne A. R., Feeley J. E., Huisman T. H., Burns C. P. Low pulse oximeter-measured hemoglobin oxygen saturations with hemoglobin Cheverly. Am J Hematol. 1998 Nov;59(3):181–184. doi: 10.1002/(sici)1096-8652(199811)59:3<181::aid-ajh1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Homi J., Levee L., Higgs D., Thomas P., Serjeant G. Pulse oximetry in a cohort study of sickle cell disease. Clin Lab Haematol. 1997 Mar;19(1):17–22. doi: 10.1046/j.1365-2257.1997.00215.x. [DOI] [PubMed] [Google Scholar]
- Lenfant C., Torrance J. D., Finch C. A. The regulation of hemoglobin affinity for oxygen in man. Trans Assoc Am Physicians. 1969;82:121–128. [PubMed] [Google Scholar]
- Lubin B. H., Pena V., Mentzer W. C., Bymun E., Bradley T. B., Packer L. Dimethyl adipimidate: a new antisickling agent. Proc Natl Acad Sci U S A. 1975 Jan;72(1):43–46. doi: 10.1073/pnas.72.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp R. A., Popp D. M., Shinpock S. G., Yang M. Y., Mural J. G., Aguinaga M. P., Kopsombut P., Roa P. D., Turner E. A., Rubin E. M. A transgenic mouse model of hemoglobin S Antilles disease. Blood. 1997 Jun 1;89(11):4204–4212. [PubMed] [Google Scholar]
- Rodgers G. P., Dover G. J., Noguchi C. T., Schechter A. N., Nienhuis A. W. Hematologic responses of patients with sickle cell disease to treatment with hydroxyurea. N Engl J Med. 1990 Apr 12;322(15):1037–1045. doi: 10.1056/NEJM199004123221504. [DOI] [PubMed] [Google Scholar]
- Rose Z. B., Liebowitz J. Direct determination of 2,3-diphosphoglycerate. Anal Biochem. 1970 May;35(1):177–180. doi: 10.1016/0003-2697(70)90023-0. [DOI] [PubMed] [Google Scholar]
- Ueda Y., Nagel R. L., Bookchin R. M. An increased Bohr effect in sickle cell anemia. Blood. 1979 Mar;53(3):472–480. [PubMed] [Google Scholar]
- Walters M. C. Bone marrow transplantation for sickle cell disease: where do we go from here? J Pediatr Hematol Oncol. 1999 Nov-Dec;21(6):467–474. doi: 10.1097/00043426-199911000-00005. [DOI] [PubMed] [Google Scholar]
- Weiss E. B., Slawsky P., Desforges J. F. Oxyhemoglobin affinity in chronic pulmonary granulomatosis (sarcoidosis) and fibrosis. Am Rev Respir Dis. 1971 Nov;104(5):694–702. doi: 10.1164/arrd.1971.104.5.694. [DOI] [PubMed] [Google Scholar]
- Young R. C., Jr, Rachal R. E., Hackney R. L., Jr, Uy C. G., Scott R. B. Smoking is a factor in causing acute chest syndrome in sickle cell anemia. J Natl Med Assoc. 1992 Mar;84(3):267–271. [PMC free article] [PubMed] [Google Scholar]
- Young R. C., Jr, Rachal R. E., Reindorf C. A., Armstrong E. M., Polk O. D., Jr, Hackney R. L., Jr, Scott R. B. Lung function in sickle cell hemoglobinopathy patients compared with healthy subjects. J Natl Med Assoc. 1988 May;80(5):509–514. [PMC free article] [PubMed] [Google Scholar]


