Abstract
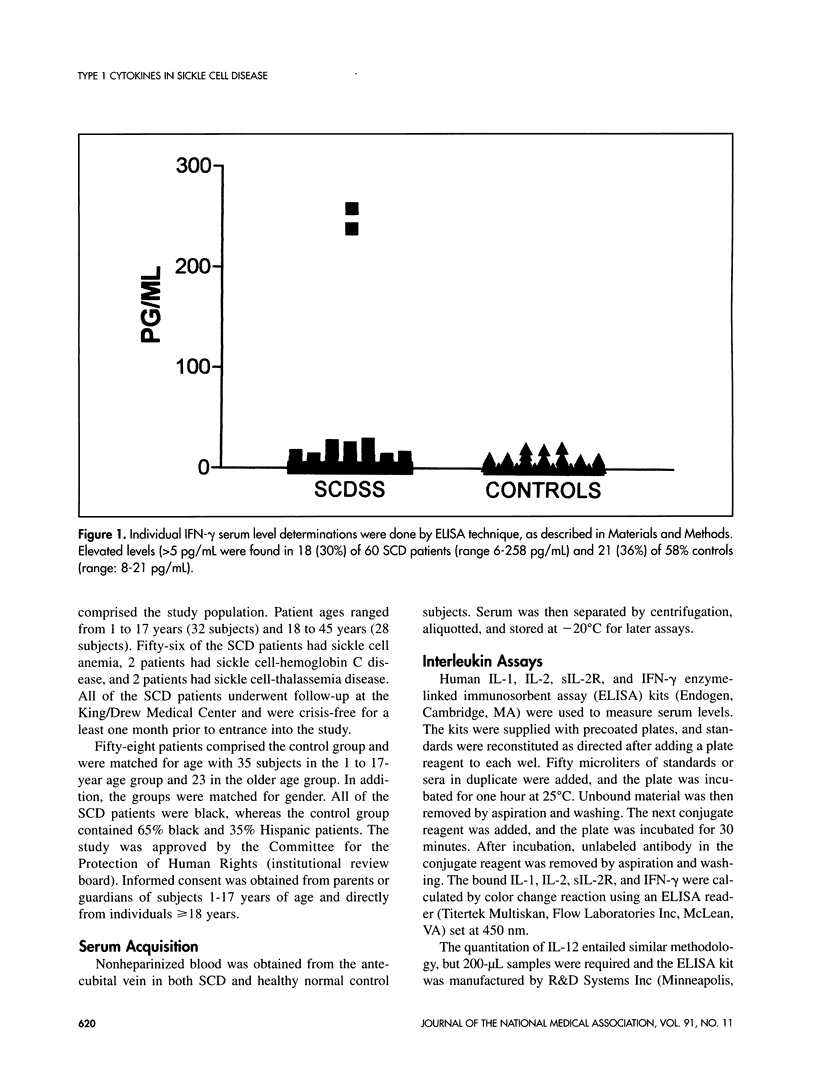
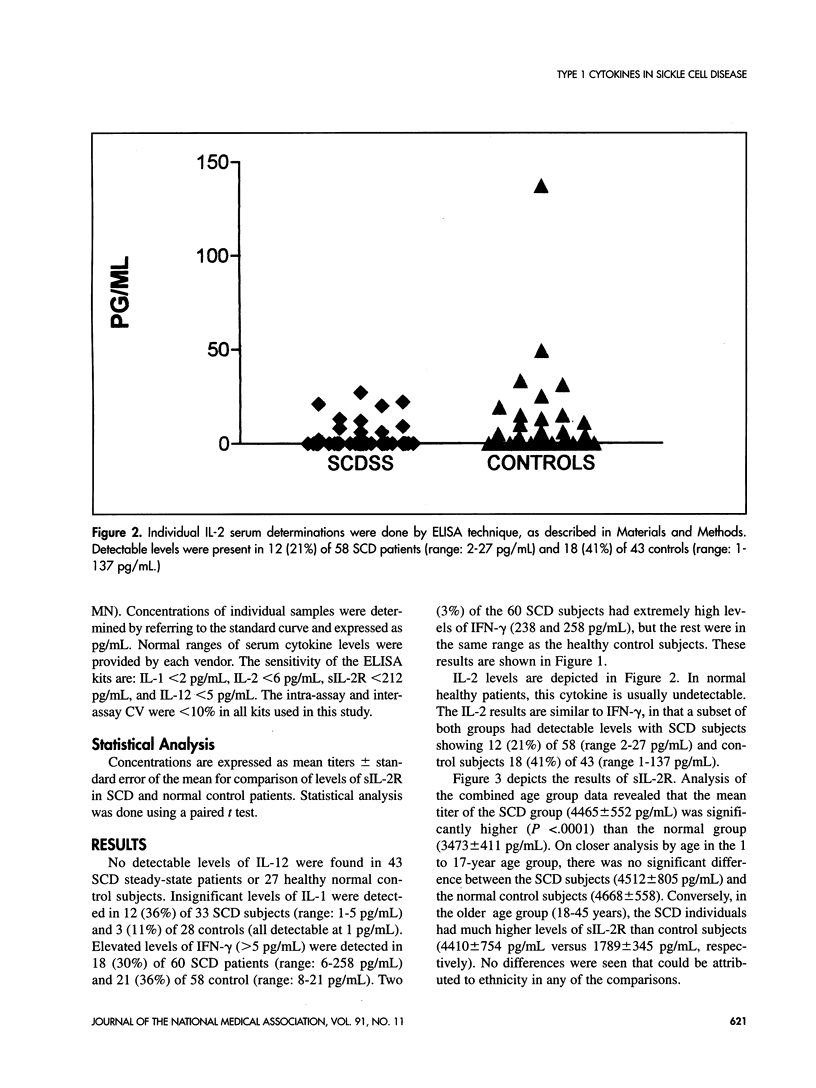
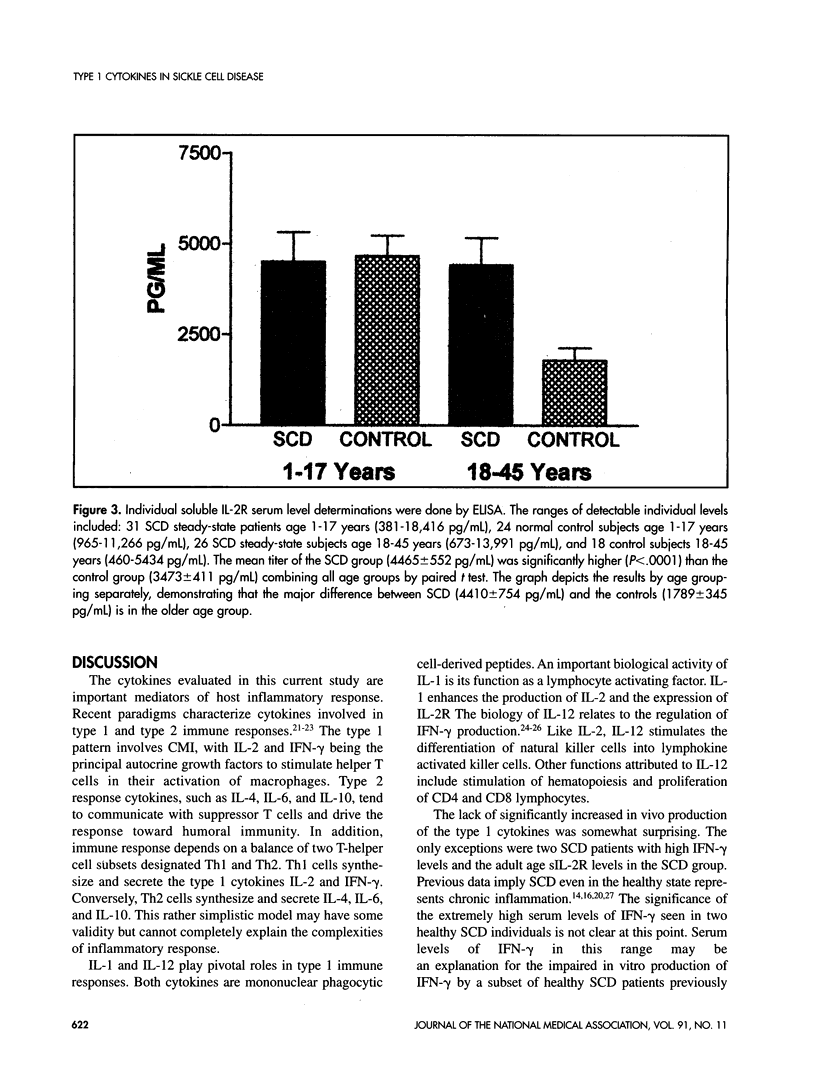
Interleukins (IL)-1, 2, 12, and interferon (IFN)-gamma, along with soluble IL-2 receptor (sIL-2R) were measured from sera obtained from healthy sickle cell disease (SCD) patients and comparable healthy control subjects. The cytokines were assessed by enzyme-linked immunosorbent assay (ELISA) in 60 SCD patients and 58 controls. No significant detectable levels of IL-1 or IL-12 were found in the sera of either group of patients. Significantly elevated levels of IFN-gamma were measured in 20 (33%) of 60 SCD patients and 21 (36%) of 58 controls. A large subset of 18 (41%) of 43 healthy controls and a smaller subset of 12 (21%) of 58 SCD demonstrated detectable levels of IL-2. The sIL-2R levels of the SCD group (4465 +/- 552 pg/mL) were significantly higher (P < .0001) than that of controls (3473 +/- 411 pg/mL). The results revealed comparable circulating levels of all type 1 cytokines in both healthy SCD and normal control subjects, with the exception of in vivo sIL-2R production. Elevated serum levels of both IL-6 and tumor necrosis factor (TNF)-alpha have been reported previously in a significant percentage of SCD steady-state subjects. These two cytokines are known to increase sIL-2R expression and may help explain the difference between the patient populations. Immune activation markers such as sIL-2R are produced by cells that mediate host responses to infection or inflammatory stimuli. The implication of higher levels of sIL-2R in SCD is not clear, but chronic parvovirus B19 infection, chronic polyclonal activation of B cells or defective regulation of antibodies are possible explanations for the elevated levels in SCD.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz N., Nishanian P., Taylor J. M., Mitsuyasu R. T., Jacobson J. M., Dezube B. J., Lederman M. M., Detels R., Fahey J. L. Stability of plasma levels of cytokines and soluble activation markers in patients with human immunodeficiency virus infection. J Infect Dis. 1999 Apr;179(4):843–848. doi: 10.1086/314673. [DOI] [PubMed] [Google Scholar]
- Bakshi S. S., Grover R., Cabezon E., Wethers D. L. Febrile episodes in children with sickle cell disease treated on an ambulatory basis. J Assoc Acad Minor Phys. 1991;2(2):80–83. [PubMed] [Google Scholar]
- Balter M. AIDS research. Cytokines move from the margins into the spotlight. Science. 1995 Apr 14;268(5208):205–206. doi: 10.1126/science.7716511. [DOI] [PubMed] [Google Scholar]
- Boghossian S. H., Wright G., Webster A. D., Segal A. W. Investigations of host defence in patients with sickle cell disease. Br J Haematol. 1985 Mar;59(3):523–531. doi: 10.1111/j.1365-2141.1985.tb07339.x. [DOI] [PubMed] [Google Scholar]
- Borish L., Rosenwasser L. J. Update on cytokines. J Allergy Clin Immunol. 1996 Mar;97(3):719–734. doi: 10.1016/s0091-6749(96)80146-1. [DOI] [PubMed] [Google Scholar]
- Bunn H. F. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997 Sep 11;337(11):762–769. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- Cetiner S., Akoğlu T. F., Kilinç Y., Akoğlu E., Kümi M. Immunological studies in sickle cell disease: comparison of homozygote mild and severe variants. Clin Immunol Immunopathol. 1989 Oct;53(1):32–39. doi: 10.1016/0090-1229(89)90098-6. [DOI] [PubMed] [Google Scholar]
- Corry J. M., Polhill R. B., Jr, Edmonds S. R., Johnston R. B., Jr Activity of the alternative complement pathway after splenectomy: comparison to activity in sickle cell disease and hypogammaglobulinemia. J Pediatr. 1979 Dec;95(6):964–969. doi: 10.1016/s0022-3476(79)80284-x. [DOI] [PubMed] [Google Scholar]
- Eng V. M., Car B. D., Schnyder B., Lorenz M., Lugli S., Aguet M., Anderson T. D., Ryffel B., Quesniaux V. F. The stimulatory effects of interleukin (IL)-12 on hematopoiesis are antagonized by IL-12-induced interferon gamma in vivo. J Exp Med. 1995 May 1;181(5):1893–1898. doi: 10.1084/jem.181.5.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey J. L. Cytokines, plasma immune activation markers, and clinically relevant surrogate markers in human immunodeficiency virus infection. Clin Diagn Lab Immunol. 1998 Sep;5(5):597–603. doi: 10.1128/cdli.5.5.597-603.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996 Dec 12;384(6609):529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- Francis R. B., Jr, Haywood L. J. Elevated immunoreactive tumor necrosis factor and interleukin-1 in sickle cell disease. J Natl Med Assoc. 1992 Jul;84(7):611–615. [PMC free article] [PubMed] [Google Scholar]
- Kuvibidila S., Gardner R., Ode D., Yu L., Lane G., Warrier R. P. Tumor necrosis factor alpha in children with sickle cell disease in stable condition. J Natl Med Assoc. 1997 Sep;89(9):609–615. [PMC free article] [PubMed] [Google Scholar]
- Magram J., Connaughton S. E., Warrier R. R., Carvajal D. M., Wu C. Y., Ferrante J., Stewart C., Sarmiento U., Faherty D. A., Gately M. K. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996 May;4(5):471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- Malavé I., Perdomo Y., Escalona E., Rodriguez E., Anchustegui M., Malavé H., Arends T. Levels of tumor necrosis factor alpha/cachectin (TNF alpha) in sera from patients with sickle cell disease. Acta Haematol. 1993;90(4):172–176. doi: 10.1159/000204452. [DOI] [PubMed] [Google Scholar]
- Moore C. M., Ehlayel M., Leiva L. E., Sorensen R. U. New concepts in the immunology of sickle cell disease. Ann Allergy Asthma Immunol. 1996 May;76(5):385–403. doi: 10.1016/S1081-1206(10)63453-9. [DOI] [PubMed] [Google Scholar]
- Moore C., Ehlayel M., Inostroza J., Leiva L. E., Kuvibidila S., Yu L., Gardner R., Ode D. L., Warrier R., Sorensen R. U. Increased circulating levels of soluble HLA class I heterodimers in patients with sickle cell disease. J Natl Med Assoc. 1998 Mar;90(3):157–163. [PMC free article] [PubMed] [Google Scholar]
- Nassonov E. L., Samsonov M. Y., Tilz G. P., Beketova T. V., Semenkova E. N., Baranov A., Wachter H., Fuchs D. Serum concentrations of neopterin, soluble interleukin 2 receptor, and soluble tumor necrosis factor receptor in Wegener's granulomatosis. J Rheumatol. 1997 Apr;24(4):666–670. [PubMed] [Google Scholar]
- Natta C. L., Outschoorn I. M. IgG2 deficiency in sickle cell anaemia. Scand J Haematol. 1984 Aug;33(2):129–134. doi: 10.1111/j.1600-0609.1984.tb02387.x. [DOI] [PubMed] [Google Scholar]
- Pearson H. A., Gallagher D., Chilcote R., Sullivan E., Wilimas J., Espeland M., Ritchey A. K. Developmental pattern of splenic dysfunction in sickle cell disorders. Pediatrics. 1985 Sep;76(3):392–397. [PubMed] [Google Scholar]
- Platt O. S., Brambilla D. J., Rosse W. F., Milner P. F., Castro O., Steinberg M. H., Klug P. P. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994 Jun 9;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- Ranney H. M. The spectrum of sickle cell disease. Hosp Pract (Off Ed) 1992 Jan 15;27(1):133-7, 141-4, 149-50 passim. doi: 10.1080/21548331.1992.11705347. [DOI] [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Fritz M. E., Biddison W. E., Boutin B., Yarchoan R., Nelson D. L. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985 Nov;135(5):3172–3177. [PubMed] [Google Scholar]
- Taylor S. C., Shacks S. J., Mitchell R. A. In vitro lymphocyte blastogenic responses and cytokine production in sickle cell disease patients with acute pneumonia. Pediatr Infect Dis J. 1996 Apr;15(4):340–344. doi: 10.1097/00006454-199604000-00011. [DOI] [PubMed] [Google Scholar]
- Taylor S. C., Shacks S. J., Qu Z., Wiley P. Type 2 cytokine serum levels in healthy sickle cell disease patients. J Natl Med Assoc. 1997 Nov;89(11):753–757. [PMC free article] [PubMed] [Google Scholar]
- Taylor S. C., Shacks S. J., Villicana S. M., Olivares J., Dinkins G. A. Interferon production in sickle cell disease. Lymphokine Res. 1990 Fall;9(3):415–423. [PubMed] [Google Scholar]
- Taylor S., Shacks S., Qu Z., Colaco V. In vitro suppression of the normal mitogenic T lymphocyte response by steady state sickle cell disease sera. Immunol Invest. 1997 Aug-Dec;26(5-7):561–568. doi: 10.3109/08820139709088540. [DOI] [PubMed] [Google Scholar]
- Taylor S., Shacks S., Villicana S., Olivares J., Dinkins G. Lymphocyte blastogenic responses in sickle cell disease. Immunol Invest. 1991 Dec;20(7):645–655. doi: 10.3109/08820139109026244. [DOI] [PubMed] [Google Scholar]
- Van Parijs L., Abbas A. K. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998 Apr 10;280(5361):243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- Wong W. Y., Overturf G. D., Powars D. R. Infection caused by Streptococcus pneumoniae in children with sickle cell disease: epidemiology, immunologic mechanisms, prophylaxis, and vaccination. Clin Infect Dis. 1992 May;14(5):1124–1136. doi: 10.1093/clinids/14.5.1124. [DOI] [PubMed] [Google Scholar]


