Abstract
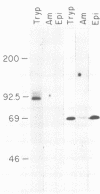
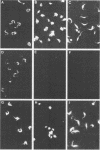
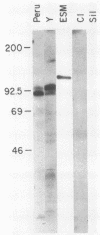
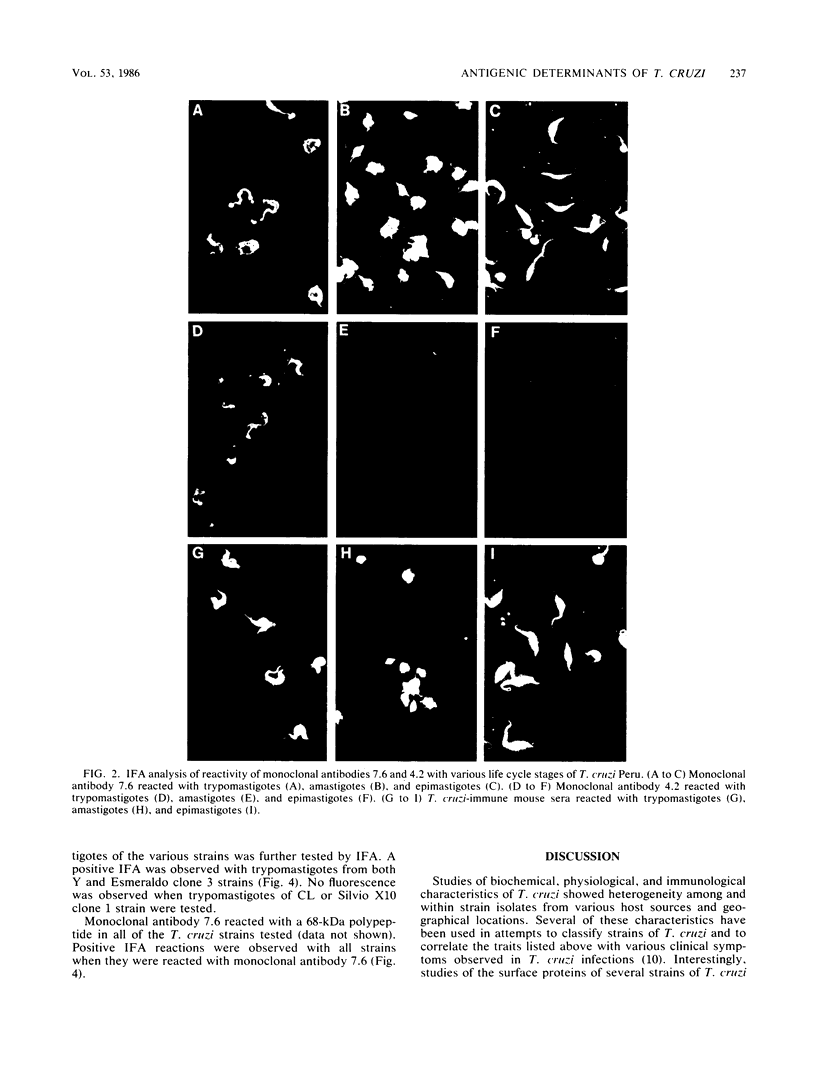
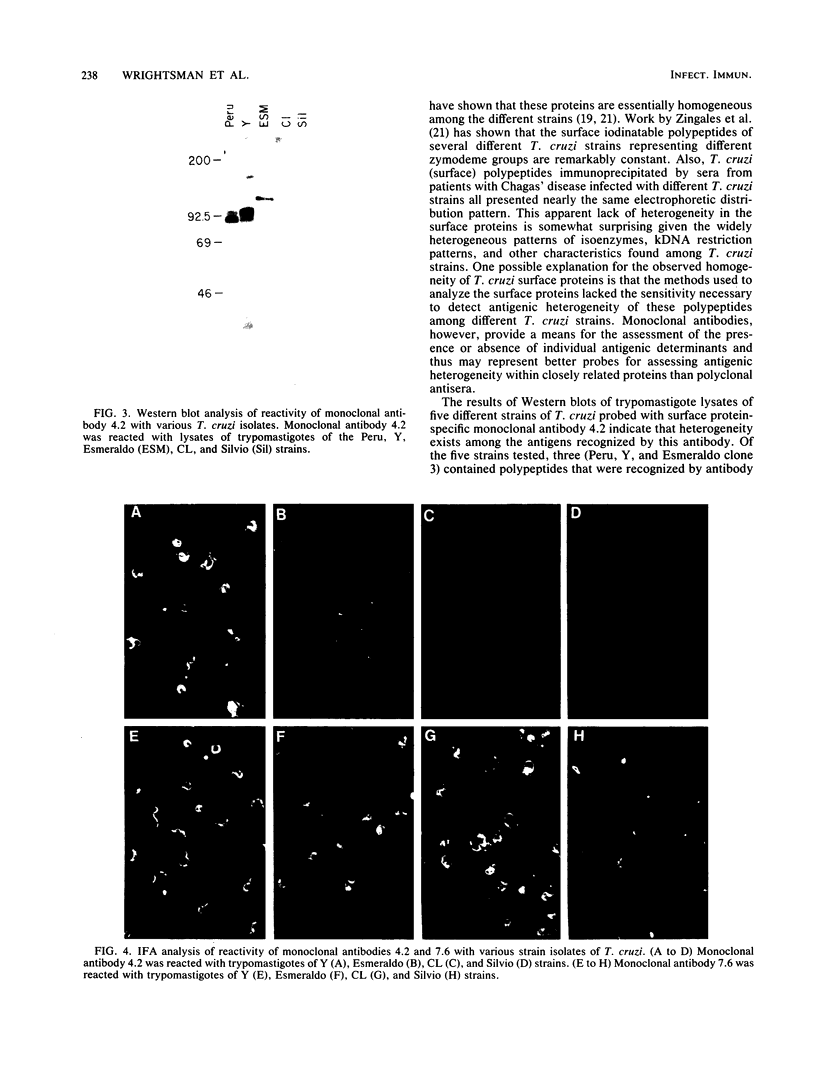
Monoclonal antibodies reactive with the surface antigens of the Peru strain of Trypanosoma cruzi were analyzed by Western blots and immunofluorescence assays to determine their reactivity with three life cycle stages and five strain isolates of T. cruzi. One monoclonal antibody, 7.6, recognized a 68-kilodalton (kDa) polypeptide in Western blots of Peru strain trypomastigotes, epimastigotes, and amastigotes. A 68-kDa polypeptide was also detected by monoclonal antibody 7.6 in trypomastigotes of the CL and Y strains and in the clonal isolates Esmeraldo clone 3 and Silvio X10 clone 1. Positive immunofluorescence results were obtained for all life cycle stages of the five strains that were reacted with monoclonal antibody 7.6, thus indicating that the antigen recognized by monoclonal antibody 7.6 is universally present in all T. cruzi strains tested. In contrast, monoclonal antibody 4.2 reacted with a polypeptide doublet of 90 and 105 kDa in Western blots of Peru strain trypomastigotes, but it did not detect these antigens in epimastigotes or amastigotes. The same polypeptide doublet of 90 and 105 kDa was also detected in Western blots of Y strain trypomastigotes; however, no bands were detected in blots of strain CL or isolate Silvio X10 clone 1 trypomastigotes. In blots of Esmeraldo clone 3 trypomastigotes, a single band of 130 kDa was detected by monoclonal antibody 4.2. In immunofluorescence assays of monoclonal antibody 4.2, positive reactions were obtained only with trypomastigotes of Peru, Y, and Esmeraldo clone 3 strains. Thus, monoclonal antibody 4.2 recognizes a trypomastigote-specific antigen which is not universally present on all strains of T. cruzi.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo F. G., Sharma S. D., Tsai V., Cox P., Remington J. S. Monoclonal antibodies to stages of Trypanosoma cruzi: characterization and use for antigen detection. Infect Immun. 1982 Jul;37(1):344–349. doi: 10.1128/iai.37.1.344-349.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard C. A., Wrightsman R. A., Manning J. E. Identification of monoclonal antibodies against the trypomastigote stage of Trypanosoma cruzi by use of iminobiotinylated surface polypeptides. Mol Biochem Parasitol. 1985 Aug;16(2):199–212. doi: 10.1016/0166-6851(85)90087-8. [DOI] [PubMed] [Google Scholar]
- Chao D., Dusanic D. G. Monoclonal antibodies to metacyclic stage antigens of Trypanosoma cruzi. Am J Trop Med Hyg. 1985 Jul;34(4):694–701. doi: 10.4269/ajtmh.1985.34.694. [DOI] [PubMed] [Google Scholar]
- Doyle P. S., Dvorak J. A., Engel J. C. Trypanosoma cruzi: quantification and analysis of the infectivity of cloned stocks. J Protozool. 1984 May;31(2):280–283. doi: 10.1111/j.1550-7408.1984.tb02961.x. [DOI] [PubMed] [Google Scholar]
- Dragon E. A., Brothers V. M., Wrightsman R. A., Manning J. A Mr 90 000 surface polypeptide of Trypanosoma cruzi as a candidate for a Chagas' disease diagnostic antigen. Mol Biochem Parasitol. 1985 Sep;16(3):213–229. doi: 10.1016/0166-6851(85)90065-9. [DOI] [PubMed] [Google Scholar]
- Dvorak J. A. The natural heterogeneity of Trypanosoma cruzi: biological and medical implications. J Cell Biochem. 1984;24(4):357–371. doi: 10.1002/jcb.240240406. [DOI] [PubMed] [Google Scholar]
- Engel J. C., Doyle P. S., Dvorak J. A. Trypanosoma cruzi: biological characterization of clones derived from chronic chagasic patients. II. Quantitative analysis of the intracellular cycle. J Protozool. 1985 Feb;32(1):80–83. doi: 10.1111/j.1550-7408.1985.tb03017.x. [DOI] [PubMed] [Google Scholar]
- Engel J. C., Dvorak J. A., Segura E. L., Crane M. S. Trypanosoma cruzi: biological characterization of 19 clones derived from two chronic chagasic patients. I. Growth kinetics in liquid medium. J Protozool. 1982 Nov;29(4):555–560. doi: 10.1111/j.1550-7408.1982.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Flint J. E., Schechter M., Chapman M. D., Miles M. A. Zymodeme and species specificities of monoclonal antibodies raised against Trypanosoma cruzi. Trans R Soc Trop Med Hyg. 1984;78(2):193–202. doi: 10.1016/0035-9203(84)90276-1. [DOI] [PubMed] [Google Scholar]
- Grant C. K., Ernisse B. J., Jarrett O., Jones F. R. Feline leukemia virus envelope gp70 of subgroups B and C defined by monoclonal antibodies with cytotoxic and neutralizing functions. J Immunol. 1983 Dec;131(6):3042–3048. [PubMed] [Google Scholar]
- Jaffe C. L., Bennett E., Grimaldi G., Jr, McMahon-Pratt D. Production and characterization of species-specific monoclonal antibodies against Leishmania donovani for immunodiagnosis. J Immunol. 1984 Jul;133(1):440–447. [PubMed] [Google Scholar]
- Miles M. A., Cedillos R. A., Póvoa M. M., de Souza A. A., Prata A., Macedo V. Do radically dissimilar Trypanosoma cruzi strains (zymodemes) cause Venezuelan and Brazilian forms of Chagas' disease? Lancet. 1981 Jun 20;1(8234):1338–1340. doi: 10.1016/s0140-6736(81)92518-6. [DOI] [PubMed] [Google Scholar]
- Miles M. A., Toye P. J., Oswald S. C., Godfrey D. G. The identification by isoenzyme patterns of two distinct strain-groups of Trypanosoma cruzi, circulating independently in a rural area of Brazil. Trans R Soc Trop Med Hyg. 1977;71(3):217–225. doi: 10.1016/0035-9203(77)90012-8. [DOI] [PubMed] [Google Scholar]
- Morel C., Chiari E., Camargo E. P., Mattei D. M., Romanha A. J., Simpson L. Strains and clones of Trypanosoma cruzi can be characterized by pattern of restriction endonuclease products of kinetoplast DNA minicircles. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6810–6814. doi: 10.1073/pnas.77.11.6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellano E., Cazzulo J. J. Purification and regulatory properties of the NADP-linked malic enzyme for Crithidia fasciculata. Mol Biochem Parasitol. 1981 May;3(1):1–11. doi: 10.1016/0166-6851(81)90072-4. [DOI] [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J. R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980 Aug;29(2):724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postan M., Dvorak J. A., McDaniel J. P. Studies of Trypanosoma cruzi clones in inbred mice. I. A comparison of the course of infection of C3H/HEN- mice with two clones isolated from a common source. Am J Trop Med Hyg. 1983 May;32(3):497–506. doi: 10.4269/ajtmh.1983.32.497. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J., Thomas J. A., Twomey C. E. The growth of Trypanosoma cruzi in human diploid cells for the production of trypomastigotes. Parasitology. 1980 Feb;80(1):153–162. doi: 10.1017/s0031182000000615. [DOI] [PubMed] [Google Scholar]
- Snary D. Trypanosoma cruzi: antigenic invariance of the cell surface glycoprotein. Exp Parasitol. 1980 Feb;49(1):68–77. doi: 10.1016/0014-4894(80)90057-0. [DOI] [PubMed] [Google Scholar]
- Zingales B., Abuin G., Romanha A. J., Chiari E., Colli W. Surface antigens of stocks and clones of Trypanosoma cruzi isolated from humans. Acta Trop. 1984 Mar;41(1):5–16. [PubMed] [Google Scholar]