Abstract
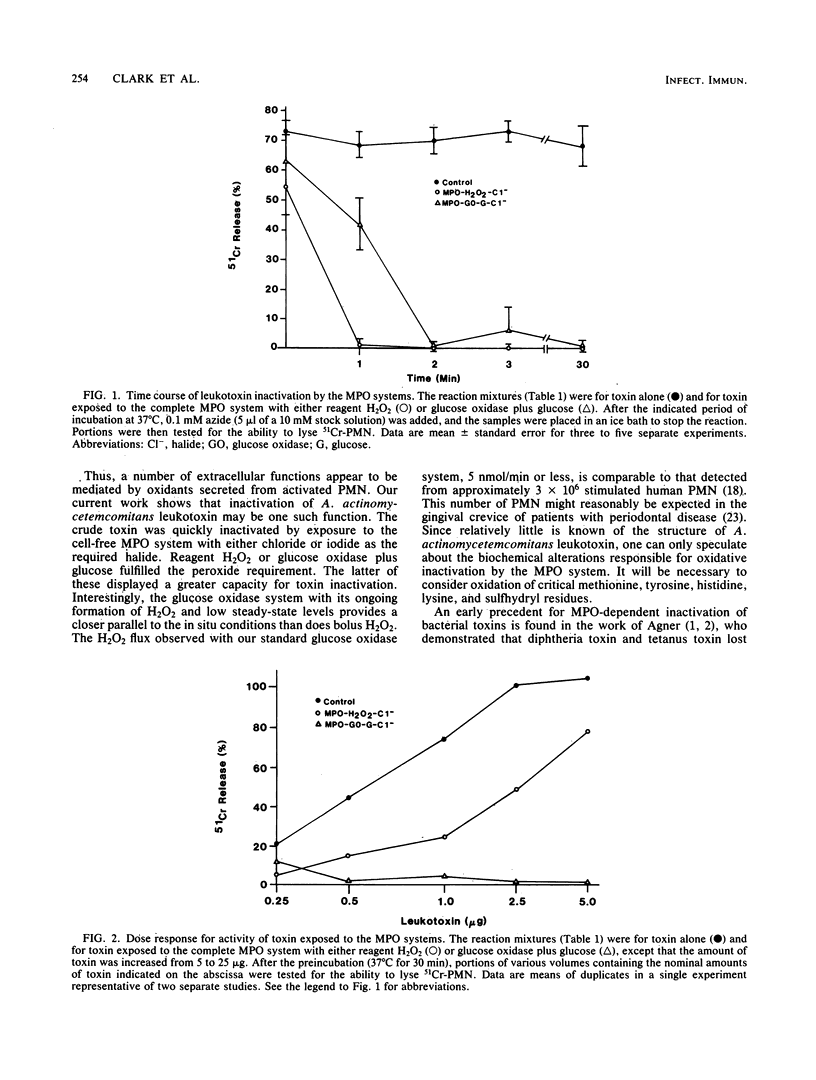
The leukotoxin of Actinobacillus actinomycetemcomitans has been implicated in the pathogenesis of inflammatory periodontal disease. We examined a potential mechanism for detoxification of this microbial product by the neutrophil myeloperoxidase system. Exposure to myeloperoxidase, H2O2, and a halide resulted in marked inactivation of leukotoxin, an effect which required each component of the myeloperoxidase system. Toxin inactivation was blocked by agents which inhibit heme enzymes (azide, cyanide) or degrade H2O2 (catalase). Reagent H2O2 could be replaced by the peroxide-generating enzyme system glucose oxidase plus glucose. The latter system, in fact, was more potent than reagent H2O2 in terms of the capacity to inactivate high concentrations of toxin. Toxin inactivation was complete within 1 to 2 min at 37 degrees C. These observations suggest a possible role for oxidative inactivation of leukotoxin by secretory products of neutrophils.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGNER K. Studies on peroxidative detoxification of purified diphtheria toxin. J Exp Med. 1950 Oct 1;92(4):337–347. doi: 10.1084/jem.92.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehni P. C., Tsai C. C., McArthur W. P., Hammond B. F., Shenker B. J., Taichman N. S. Leukotoxic activity in different strains of the bacterium Actinobacillus actinomycetemcomitans isolated from juvenile periodontitis in man. Arch Oral Biol. 1981;26(8):671–676. doi: 10.1016/0003-9969(81)90164-3. [DOI] [PubMed] [Google Scholar]
- Baehni P., Tsai C. C., McArthur W. P., Hammond B. F., Taichman N. S. Interaction of inflammatory cells and oral microorganisms. VIII. Detection of leukotoxic activity of a plaque-derived gram-negative microorganism. Infect Immun. 1979 Apr;24(1):233–243. doi: 10.1128/iai.24.1.233-243.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Carp H., Janoff A. Potential mediator of inflammation. Phagocyte-derived oxidants suppress the elastase-inhibitory capacity of alpha 1-proteinase inhibitor in vitro. J Clin Invest. 1980 Nov;66(5):987–995. doi: 10.1172/JCI109968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Chemotactic factor inactivation by the myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest. 1979 Oct;64(4):913–920. doi: 10.1172/JCI109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Role of the myeloperoxidase-H2O2-halide system in concanavalin A-induced tumor cell killing by human neutrophils. J Immunol. 1979 Jun;122(6):2605–2610. [PubMed] [Google Scholar]
- Clark R. A. Neutrophil iodination reaction induced by fluoride: implications for degranulation and metabolic activation. Blood. 1981 May;57(5):913–921. [PubMed] [Google Scholar]
- Clark R. A., Stone P. J., El Hag A., Calore J. D., Franzblau C. Myeloperoxidase-catalyzed inactivation of alpha 1-protease inhibitor by human neutrophils. J Biol Chem. 1981 Apr 10;256(7):3348–3353. [PubMed] [Google Scholar]
- Clark R. A., Szot S. Chemotactic factor inactivation by stimulated human neutrophils mediated by myeloperoxidase-catalyzed methionine oxidation. J Immunol. 1982 Apr;128(4):1507–1513. [PubMed] [Google Scholar]
- Gallin J. I., Clark R. A., Kimball H. R. Granulocyte chemotaxis: an improved in vitro assay employing 51 Cr-labeled granulocytes. J Immunol. 1973 Jan;110(1):233–240. [PubMed] [Google Scholar]
- Henderson W. R., Klebanoff S. J. Leukotriene production and inactivation by normal, chronic granulomatous disease and myeloperoxidase-deficient neutrophils. J Biol Chem. 1983 Nov 25;258(22):13522–13527. [PubMed] [Google Scholar]
- Homan-Müller J. W., Weening R. S., Roos D. Production of hydrogen peroxide by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):198–207. [PubMed] [Google Scholar]
- Klebanoff S. J., Clark R. A. Hemolysis and iodination of erythrocyte components by a myeloperoxidase-mediated system. Blood. 1975 May;45(5):699–707. [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- Klinkhamer J. M. Quantitative evaluation of gingivitis and periodontal disease. I. The orogranulocytic migratory rate. Periodontics. 1968 Oct;6(5):207–211. [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Enzymatic inactivation of human alpha-1-proteinase inhibitor by neutrophil myeloperoxidase. Biochem Biophys Res Commun. 1979 May 28;88(2):402–409. doi: 10.1016/0006-291x(79)92062-x. [DOI] [PubMed] [Google Scholar]
- Odajima T. Oxidative destruction of the microbial metabolite aflatoxin by the myeloperoxidase-hydrogen peroxide-chloride system. Arch Oral Biol. 1981;26(4):339–340. doi: 10.1016/0003-9969(81)90057-1. [DOI] [PubMed] [Google Scholar]
- Ooi W., Levine H. G., LaMont J. T., Clark R. A. Inactivation of Clostridium difficile cytotoxin by the neutrophil myeloperoxidase system. J Infect Dis. 1984 Feb;149(2):215–219. doi: 10.1093/infdis/149.2.215. [DOI] [PubMed] [Google Scholar]
- Taichman N. S., Wilton J. M. Leukotoxicity of an extract from Actinobacillus actinomycetemcomitans for human gingival polymorphonuclear leukocytes. Inflammation. 1981 Mar;5(1):1–12. doi: 10.1007/BF00910774. [DOI] [PubMed] [Google Scholar]
- Tsai C. C., McArthur W. P., Baehni P. C., Evian C., Genco R. J., Taichman N. S. Serum neutralizing activity against Actinobacillus actinomycetemcomitans leukotoxin in juvenile periodontitis. J Clin Periodontol. 1981 Aug;8(4):338–348. doi: 10.1111/j.1600-051x.1981.tb02043.x. [DOI] [PubMed] [Google Scholar]
- Tsai C. C., McArthur W. P., Baehni P. C., Hammond B. F., Taichman N. S. Extraction and partial characterization of a leukotoxin from a plaque-derived Gram-negative microorganism. Infect Immun. 1979 Jul;25(1):427–439. doi: 10.1128/iai.25.1.427-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. C., Shenker B. J., DiRienzo J. M., Malamud D., Taichman N. S. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect Immun. 1984 Feb;43(2):700–705. doi: 10.1128/iai.43.2.700-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M. F., Denison R. C. Oxidation of n-formyl methionyl chemotactic peptide by human neutrophils. J Immunol. 1981 Apr;126(4):1387–1389. [PubMed] [Google Scholar]
- Zambon J. J. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985 Jan;12(1):1–20. doi: 10.1111/j.1600-051x.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]



