Abstract
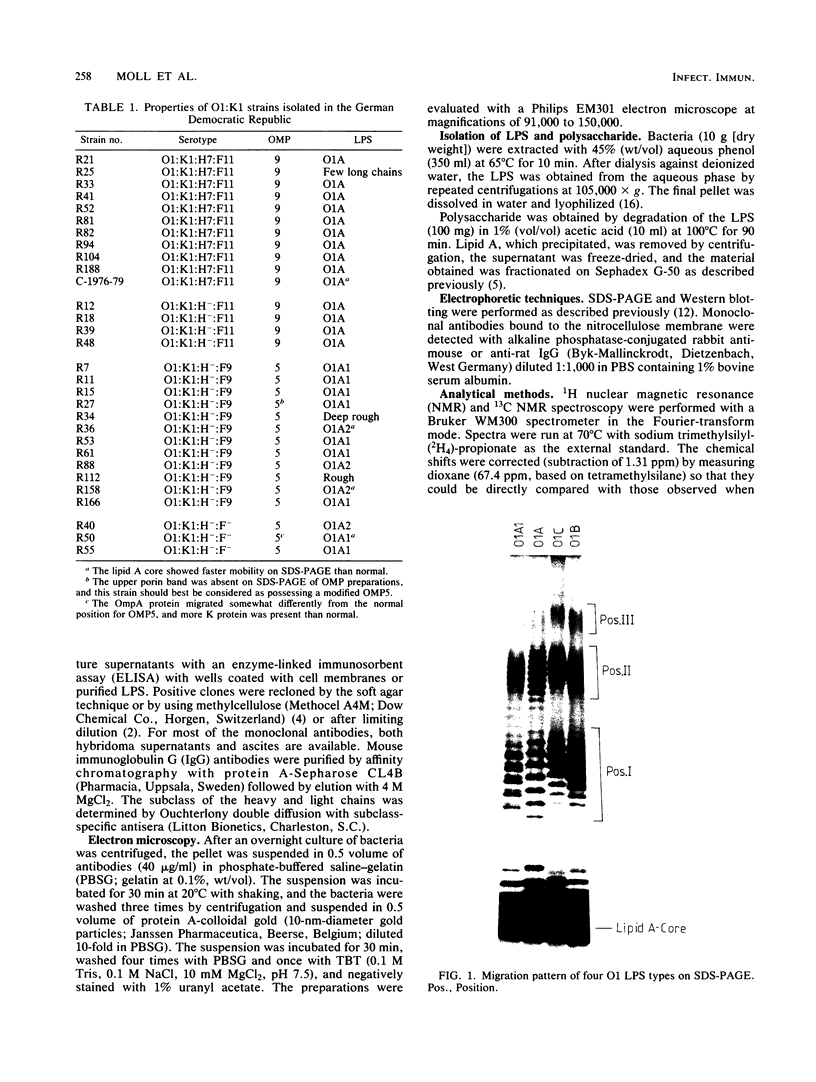
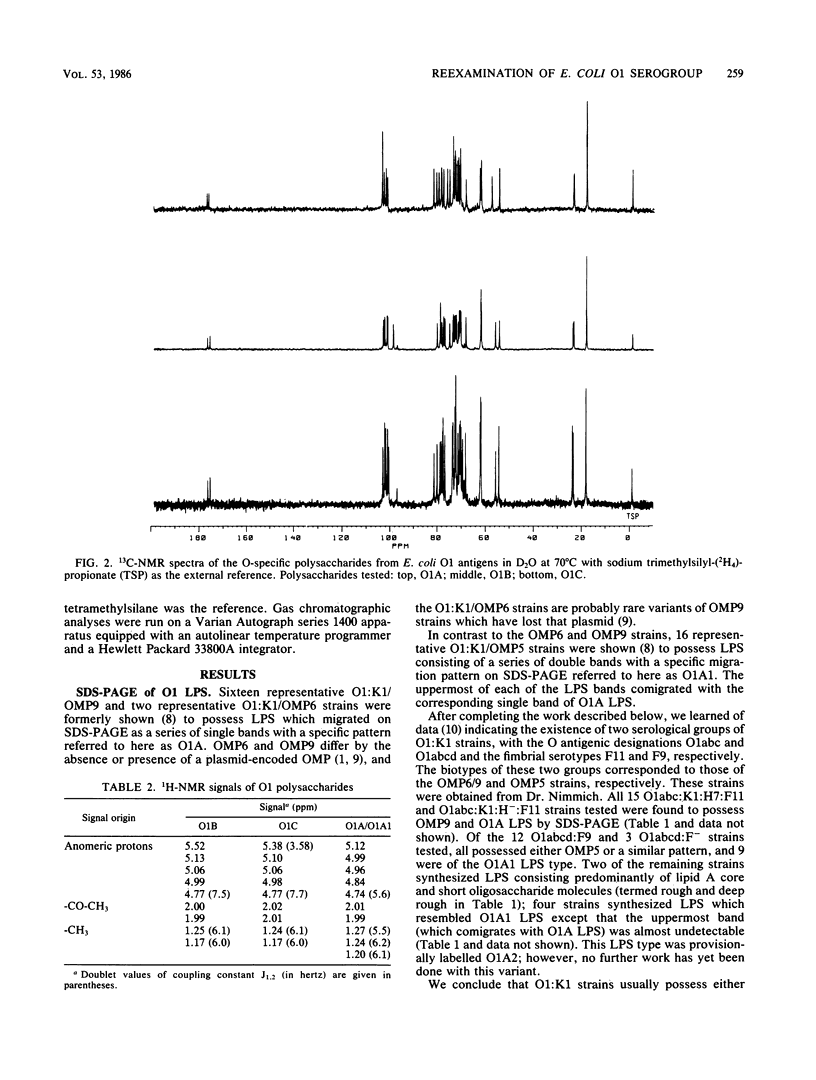
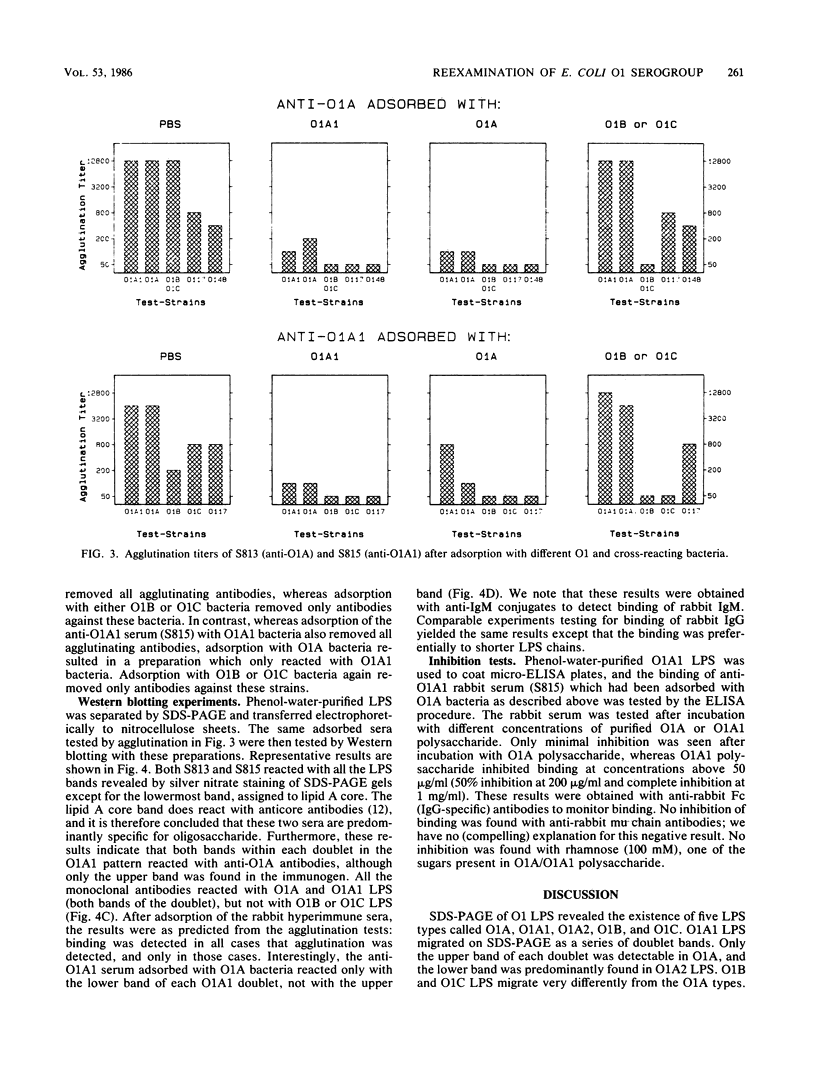
A total of 64 Escherichia coli strains of the O1 serogroup were tested for the migration pattern of their lipopolysaccharides (LPS) on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. O1:K1 and O1:K51 strains of the OMP5 outer membrane protein pattern possessed LPS with a doublet pattern (O1A1) or the lowermost band of the O1A1 doublet (O1A2). O1:K1 strains of the OMP9 pattern possessed LPS referred to as O1A, which corresponded to the uppermost band of the O1A1 doublet pattern. A few O1:K? strains possessed LPS of different migration patterns (O1B and O1C). O1A and O1A1 LPS were indistinguishable by chemical techniques, and both reacted with each of 10 different monoclonal antibodies tested. However, O1A1 had an additional epitope within the additional band in each doublet, as demonstrated by adsorption experiments with hyperimmune rabbit sera followed by Western blotting. Furthermore, purified polysaccharide from O1A bacteria was incapable of inhibition in enzyme-linked immunosorbent assays performed with O1A1 LPS as antigen and adsorbed, specific anti-O1A1 antibodies, whereas O1A1 polysaccharide inhibited this reaction. O1B and O1C LPS differed in all respects tested, including chemical composition, from O1A and O1A1 LPS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruyn A., Anteunis M. 1H-N.m.r. study of L-rhamnose, methyl alpha-L-rhamnopyranoside, and 4-o-beta-D-galactopranosyl-L-rhamnose in deuterium oxide. Carbohydr Res. 1976 Mar;47(1):158–163. doi: 10.1016/s0008-6215(00)83559-4. [DOI] [PubMed] [Google Scholar]
- Jann B., Reske K., Jann K. Heterogeneity of lipopolysaccharides. Analysis of polysaccharide chain lengths by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Eur J Biochem. 1975 Dec 1;60(1):239–246. doi: 10.1111/j.1432-1033.1975.tb20996.x. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kusecek B., Wloch H., Mercer A., Vaisänen V., Pluschke G., Korhonen T., Achtman M. Lipopolysaccharide, capsule, and fimbriae as virulence factors among O1, O7, O16, O18, or O75 and K1, K5, or K100 Escherichia coli. Infect Immun. 1984 Jan;43(1):368–379. doi: 10.1128/iai.43.1.368-379.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer A. A., Morelli G., Heuzenroeder M., Kamke M., Achtman M. Conservation of plasmids among Escherichia coli K1 isolates of diverse origins. Infect Immun. 1984 Dec;46(3):649–657. doi: 10.1128/iai.46.3.649-657.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmich W., Zingler G. Biochemical characteristics, phage patterns, and O1 factor analysis of Escherichia coli O1:K1:H7:F11 and O1:K1:H-:F9 strains isolated from patients with urinary tract infections. Med Microbiol Immunol. 1984;173(2):75–85. doi: 10.1007/BF02124821. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Moll A., Kusecek B., Achtman M. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and monoclonal antibodies as tools for the subgrouping of Escherichia coli lipopolysaccharide O18 and O23 antigens. Infect Immun. 1986 Jan;51(1):286–293. doi: 10.1128/iai.51.1.286-293.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Ulisko I. N., Andronova N. I. Partsial'nyi sostav O-antigenov ésherikhii nekotorykh O-grupp. Zh Mikrobiol Epidemiol Immunobiol. 1982 Jun;(6):40–44. [PubMed] [Google Scholar]




