Abstract
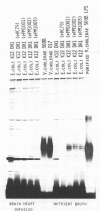
The gene clusters that determine the biosynthesis of both the Inaba and Ogawa serotypes of the O antigen of the lipopolysaccharide of Vibrio cholerae were cloned and expressed in Escherichia coli K-12. Restriction analysis of the clones demonstrated that about 15 kilobases were common to all clones and a further 5 kilobases were common to the Ogawa clones. The O antigens expressed by E. coli K-12 had the specificity of V. cholerae. Antibodies raised against E. coli K-12 that harbor one of these clones, pPM1001 (Inaba), were as highly protective in the infant mouse model system as were antibodies to V. cholerae itself. Introduction of such clones into suitable carrier strains could be expected to produce a good oral immunogen against cholera.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaicumpa W., Rowley D. Experimental cholera in infant mice: protective effects of antibody. J Infect Dis. 1972 May;125(5):480–485. doi: 10.1093/infdis/125.5.480. [DOI] [PubMed] [Google Scholar]
- Clements M. L., Levine M. M., Young C. R., Black R. E., Lim Y. L., Robins-Browne R. M., Craig J. P. Magnitude, kinetics, and duration of vibriocidal antibody responses in North Americans after ingestion of Vibrio cholerae. J Infect Dis. 1982 Apr;145(4):465–473. doi: 10.1093/infdis/145.4.465. [DOI] [PubMed] [Google Scholar]
- Collins J., Hohn B. Cosmids: a type of plasmid gene-cloning vector that is packageable in vitro in bacteriophage lambda heads. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4242–4246. doi: 10.1073/pnas.75.9.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarosa E. J., Sanati A., Saghari H., Feeley J. C. Multiple serotypes of vibrio cholerae isolated from a case of cholera. Evidence suggesting in-vivo mutation. Lancet. 1967 Mar 25;1(7491):646–648. doi: 10.1016/s0140-6736(67)92542-1. [DOI] [PubMed] [Google Scholar]
- Guidolin A., Manning P. A. Bacteriophage CP-T1 of Vibrio cholerae. Identification of the cell surface receptor. Eur J Biochem. 1985 Nov 15;153(1):89–94. doi: 10.1111/j.1432-1033.1985.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hisatsune K., Kondo S. Lipopolysaccharides of R mutants isolated from Vibria cholerae. Biochem J. 1980 Jan 1;185(1):77–81. doi: 10.1042/bj1850077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann A., Schmidt G., Rowley D. Intestinal and serum antibody responses in mice after oral immunization with Salmonella, Escherichia coli, and Salmonella-Escherichia coli hybrid strains. Infect Immun. 1979 Jul;25(1):27–33. doi: 10.1128/iai.25.1.27-33.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Levinthal M., Nikaido H. Consequences of deletion mutations joining two operons of opposite polarity. J Mol Biol. 1969 Jun 28;42(3):511–520. doi: 10.1016/0022-2836(69)90239-3. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Bartowsky E. J., Leavesly D. I., Hackett J. A., Heuzenroeder M. W. Molecular cloning using immune sera of a 22-kDal minor outer membrane protein of Vibrio cholerae. Gene. 1985;34(1):95–103. doi: 10.1016/0378-1119(85)90299-9. [DOI] [PubMed] [Google Scholar]
- Neoh S. H., Rowley D. The antigens of Vibrio cholerae involved in the vibriocidal action of antibody and complement. J Infect Dis. 1970 May;121(5):505–513. doi: 10.1093/infdis/121.5.505. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Levinthal M., Nikaido K., Nakane K. Extended deletions in the histidine-rough-B region of the Salmonella chromosome. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1825–1832. doi: 10.1073/pnas.57.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- Parker C., Gauthier D., Tate A., Richardson K., Romig W. R. Expanded linkage map of Vibrio cholerae. Genetics. 1979 Feb;91(2):191–214. doi: 10.1093/genetics/91.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond J. W., Korsch M. J., Jackson G. D. Immunochemical studies to the O-antigens of vibrio cholerae. Partial characterization of an acid-labile antigenic determinant. Aust J Exp Biol Med Sci. 1973 Apr;51(2):229–235. doi: 10.1038/icb.1973.20. [DOI] [PubMed] [Google Scholar]
- Redmond J. W. The 4-amino sugars present in the lipopolysaccharides of vibro cholerae and related vibrios. Biochim Biophys Acta. 1978 Sep 6;542(3):378–384. doi: 10.1016/0304-4165(78)90369-0. [DOI] [PubMed] [Google Scholar]
- Redmond J. W. The structure of the O-antigenic side chain of the lipopolysaccharide of Vibrio cholerae 569B (Inaba). Biochim Biophys Acta. 1979 May 1;584(2):346–352. doi: 10.1016/0304-4165(79)90280-0. [DOI] [PubMed] [Google Scholar]
- Sack R. B., Miller C. E. Progressive changes of Vibrio serotypes in germ-free mice infected with Vibrio cholerae. J Bacteriol. 1969 Sep;99(3):688–695. doi: 10.1128/jb.99.3.688-695.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakazaki R., Tamura K. Somatic antigen variation in Vibrio cholerae. Jpn J Med Sci Biol. 1971 Apr;24(2):93–100. doi: 10.7883/yoken1952.24.93. [DOI] [PubMed] [Google Scholar]
- Sears S. D., Richardson K., Young C., Parker C. D., Levine M. M. Evaluation of the human immune response to outer membrane proteins of Vibrio cholerae. Infect Immun. 1984 May;44(2):439–444. doi: 10.1128/iai.44.2.439-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]