Abstract
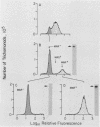
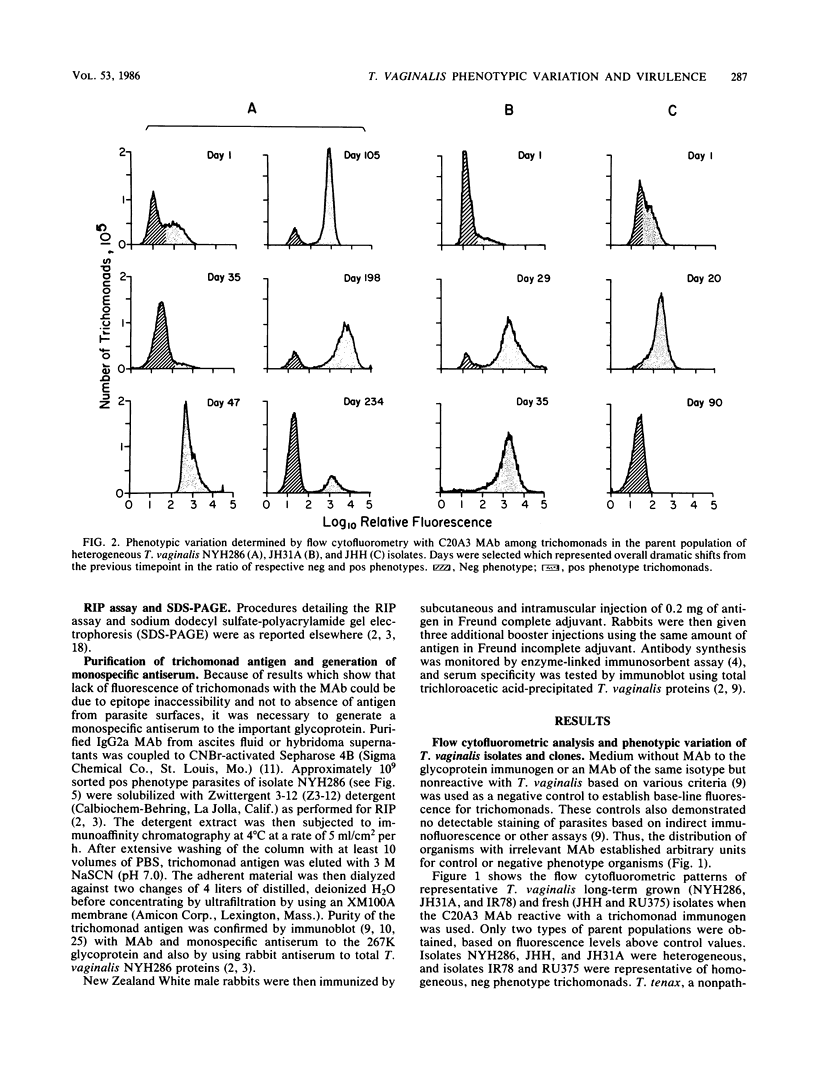
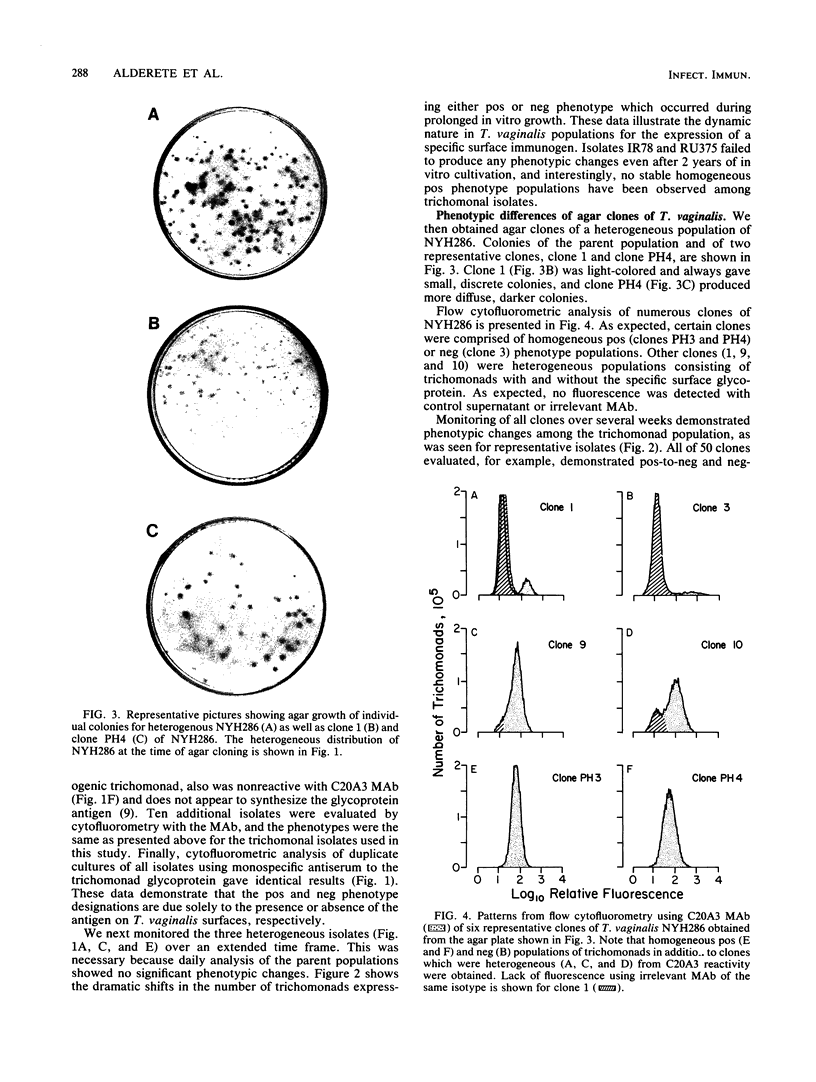
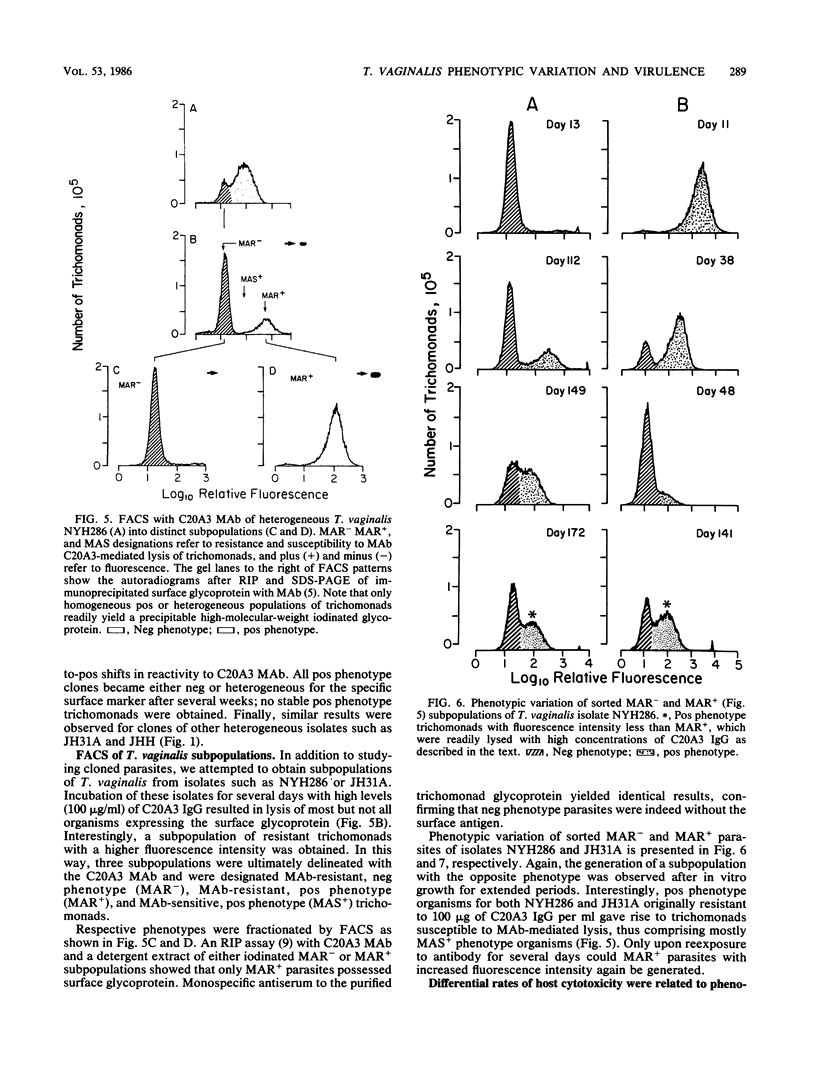
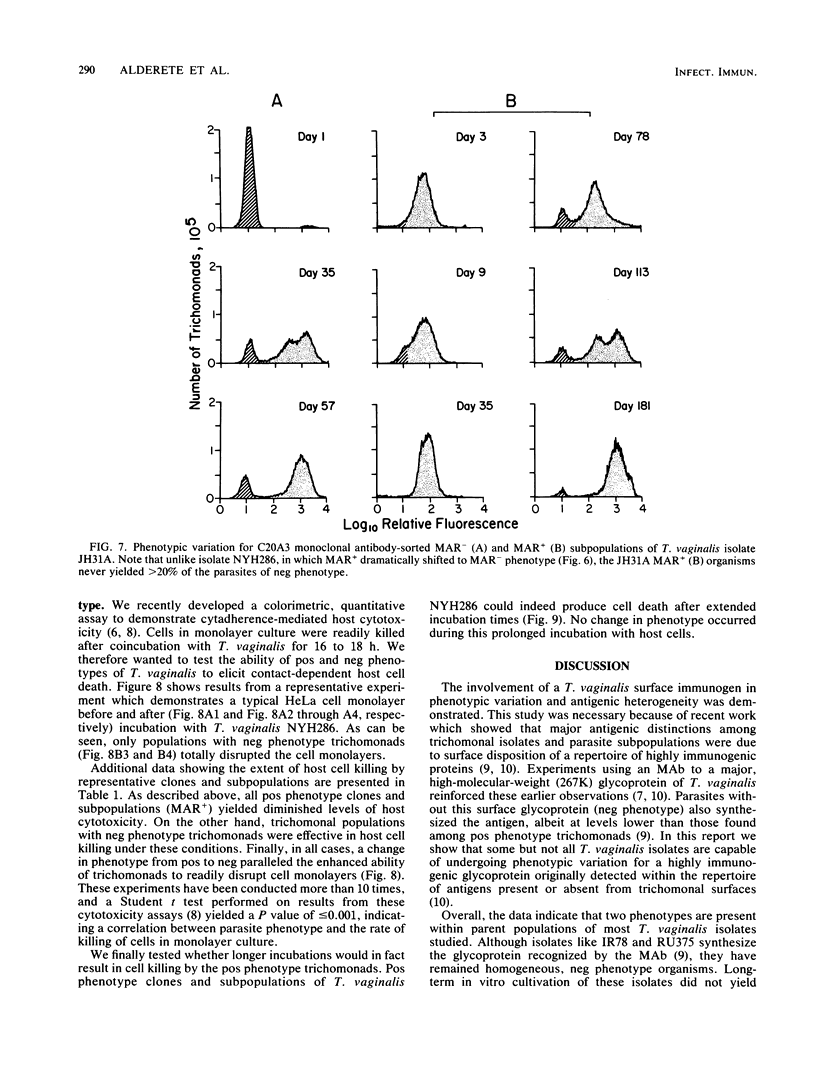
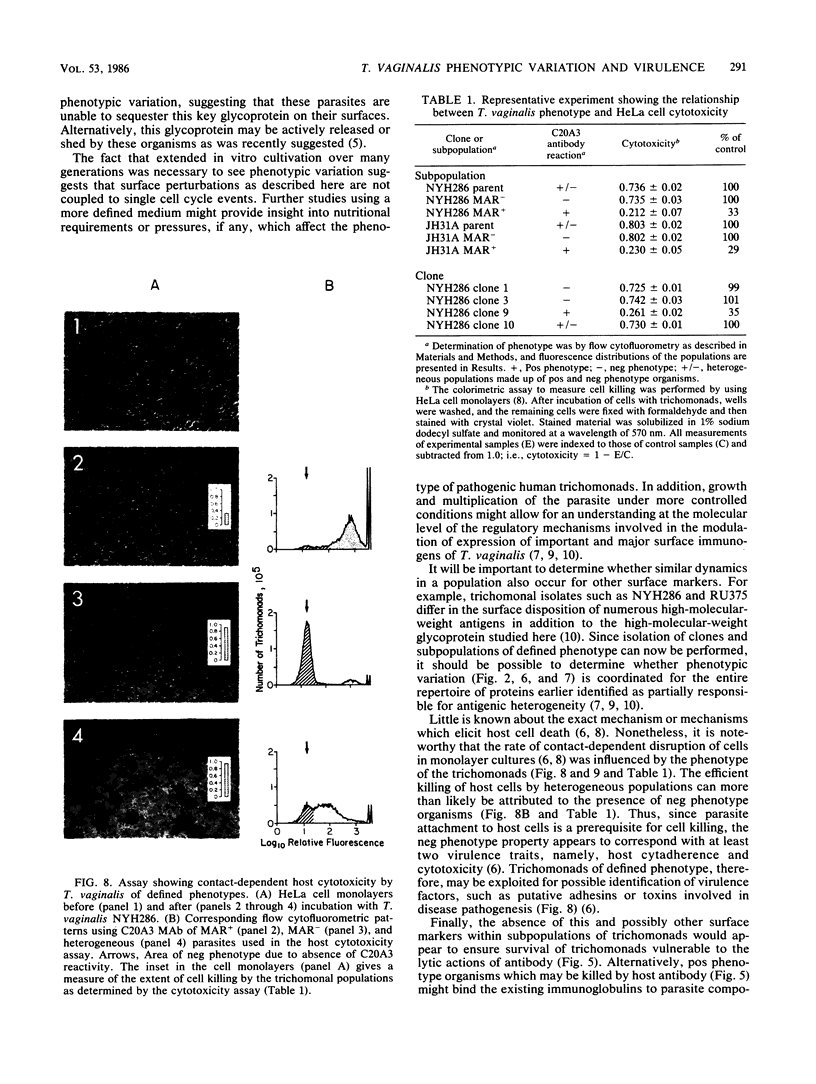
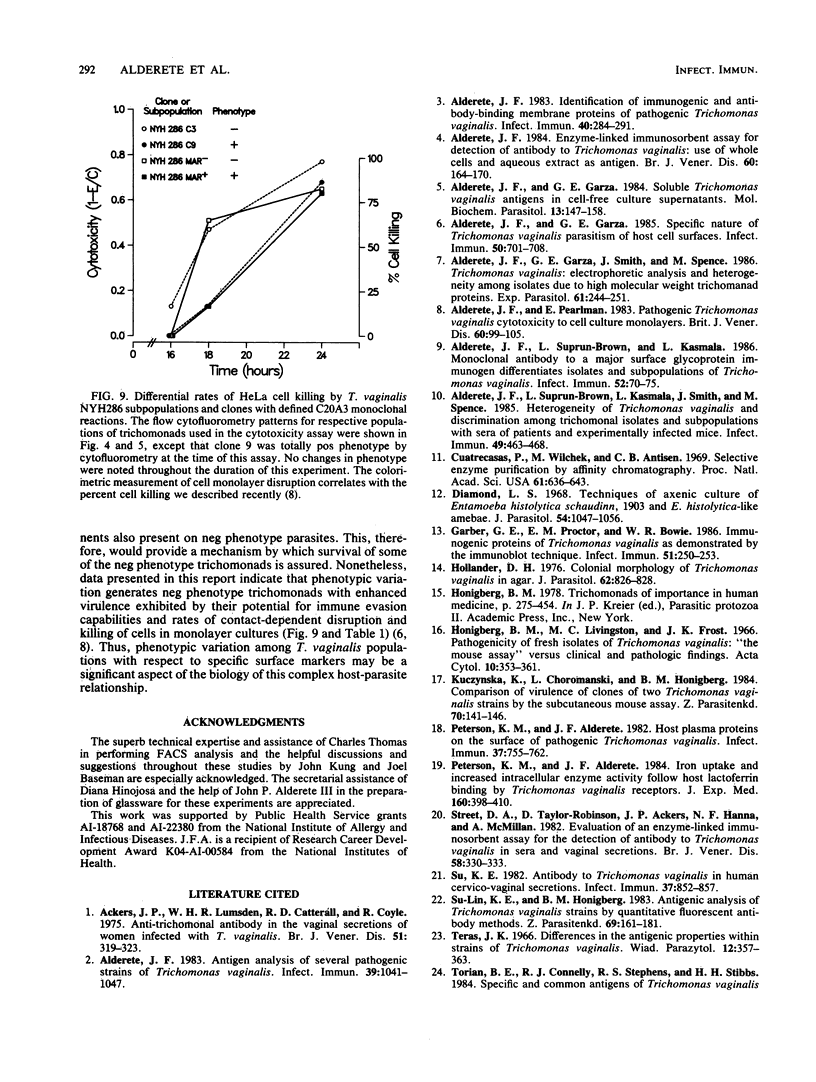
The extent and nature of heterogeneity among representative Trichomonas vaginalis isolates were evaluated by flow cytofluorometry analysis. Monoclonal antibody and monospecific antiserum to an immunodominant trichomonad surface glycoprotein with a molecular weight of 267,000 (267K glycoprotein) were used to evaluate fresh isolates (JHH and RU375) and long-term grown isolates (NYH286, IR78, and JH31A) of T. vaginalis. Isolates NYH286, JH31A, and JHH were made up of heterogeneous staining (positive [pos] phenotype) and nonstaining (negative [neg] phenotype) populations of trichomonads, whereas RU375 and IR78 were all neg phenotype parasites. Flow cytofluorometric patterns of agar clones derived from single organisms of heterogeneous isolates such as NYH286 showed populations which were either homogeneous pos or neg and also showed clones which were heterogeneous in nature containing both phenotypes. Fluorescence-activated cell sorting was also accomplished, and subpopulations of defined pos or neg phenotype were purified. Flow cytofluorometry evaluation of all isolates for an extended period revealed a phenotypic variation among all heterogeneous isolates and also for all clones and subpopulations derived from the heterogeneous isolates. On the other hand, IR78 and RU375 did not undergo phenotypic variation and have remained neg for greater than 4 years. Parasites which were nonreactive with either monoclonal antibody or monospecific antiserum to the 267K glycoprotein in flow cytofluorometry did not possess the antigen on their surface. This was determined by radioimmunoprecipitation assays using extracts of iodinated trichomonads. Finally, neg phenotype parasites yielded enhanced rates of contact-dependent cytotoxicity of host cell monolayers as compared with the pos phenotype trichomonads.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers J. P., Lumsden W. H., Catterall R. D., Coyle R. Antitrichomonal antibody in the vaginal secretions of women infected with T. vaginalis. Br J Vener Dis. 1975 Oct;51(5):319–323. doi: 10.1136/sti.51.5.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F. Antigen analysis of several pathogenic strains of Trichomonas vaginalis. Infect Immun. 1983 Mar;39(3):1041–1047. doi: 10.1128/iai.39.3.1041-1047.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F. Enzyme linked immunosorbent assay for detecting antibody to Trichomonas vaginalis: use of whole cells and aqueous extract as antigen. Br J Vener Dis. 1984 Jun;60(3):164–170. doi: 10.1136/sti.60.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Garza G. E. Soluble Trichomonas vaginalis antigens in cell-free culture supernatants. Mol Biochem Parasitol. 1984 Oct;13(2):147–158. doi: 10.1016/0166-6851(84)90109-9. [DOI] [PubMed] [Google Scholar]
- Alderete J. F., Garza G. E. Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect Immun. 1985 Dec;50(3):701–708. doi: 10.1128/iai.50.3.701-708.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Garza G., Smith J., Spence M. Trichomonas vaginalis: electrophoretic analysis and heterogeneity among isolates due to high-molecular-weight trichomonad proteins. Exp Parasitol. 1986 Apr;61(2):244–251. doi: 10.1016/0014-4894(86)90158-x. [DOI] [PubMed] [Google Scholar]
- Alderete J. F. Identification of immunogenic and antibody-binding membrane proteins of pathogenic Trichomonas vaginalis. Infect Immun. 1983 Apr;40(1):284–291. doi: 10.1128/iai.40.1.284-291.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Pearlman E. Pathogenic Trichomonas vaginalis cytotoxicity to cell culture monolayers. Br J Vener Dis. 1984 Apr;60(2):99–105. doi: 10.1136/sti.60.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Suprun-Brown L., Kasmala L. Monoclonal antibody to a major surface glycoprotein immunogen differentiates isolates and subpopulations of Trichomonas vaginalis. Infect Immun. 1986 Apr;52(1):70–75. doi: 10.1128/iai.52.1.70-75.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Suprun-Brown L., Kasmala L., Smith J., Spence M. Heterogeneity of Trichomonas vaginalis and discrimination among trichomonal isolates and subpopulations with sera of patients and experimentally infected mice. Infect Immun. 1985 Sep;49(3):463–468. doi: 10.1128/iai.49.3.463-468.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L. S. Techniques of axenic cultivation of Entamoeba histolytica Schaudinn, 1903 and E. histolytica-like amebae. J Parasitol. 1968 Oct;54(5):1047–1056. [PubMed] [Google Scholar]
- Garber G. E., Proctor E. M., Bowie W. R. Immunogenic proteins of Trichomonas vaginalis as demonstrated by the immunoblot technique. Infect Immun. 1986 Jan;51(1):250–253. doi: 10.1128/iai.51.1.250-253.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander D. H. Colonial morphology of Trichomonas vaginalis in Agar. J Parasitol. 1976 Oct;62(5):826–828. [PubMed] [Google Scholar]
- Honigberg B. M., Livingston M. C., Frost J. K. Pathogenicity of fresh isolates of Trichomonas vaginalis: "the mouse assay" versus clinical and pathologic findings. Acta Cytol. 1966 Sep-Oct;10(5):353–361. [PubMed] [Google Scholar]
- Kuczyńska K., Choromański L., Honigberg B. M. Comparison of virulence of clones of two Trichomonas vaginalis strains by the subcutaneous mouse assay. Z Parasitenkd. 1984;70(2):141–146. doi: 10.1007/BF00942215. [DOI] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Host plasma proteins on the surface of pathogenic Trichomonas vaginalis. Infect Immun. 1982 Aug;37(2):755–762. doi: 10.1128/iai.37.2.755-762.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Iron uptake and increased intracellular enzyme activity follow host lactoferrin binding by Trichomonas vaginalis receptors. J Exp Med. 1984 Aug 1;160(2):398–410. doi: 10.1084/jem.160.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street D. A., Taylor-Robinson D., Ackers J. P., Hanna N. F., McMillan A. Evaluation of an enzyme-linked immunosorbent assay for the detection of antibody to Trichomonas vaginalis in sera and vaginal secretions. Br J Vener Dis. 1982 Oct;58(5):330–333. doi: 10.1136/sti.58.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su-Lin K. E., Honigberg B. M. Antigenic analysis of Trichomonas vaginalis strains by quantitative fluorescent antibody methods. Z Parasitenkd. 1983;69(2):161–181. doi: 10.1007/BF00926952. [DOI] [PubMed] [Google Scholar]
- Su K. E. Antibody to Trichomonas vaginalis in human cervicovaginal secretions. Infect Immun. 1982 Sep;37(3):852–857. doi: 10.1128/iai.37.3.852-857.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torian B. E., Connelly R. J., Stephens R. S., Stibbs H. H. Specific and common antigens of Trichomonas vaginalis detected by monoclonal antibodies. Infect Immun. 1984 Jan;43(1):270–275. doi: 10.1128/iai.43.1.270-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warton A., Honigberg B. M. Lectin analysis of surface saccharides in two Trichomonas vaginalis strains differing in pathogenicity. J Protozool. 1980 Nov;27(4):410–419. doi: 10.1111/j.1550-7408.1980.tb05386.x. [DOI] [PubMed] [Google Scholar]
- Wartoń A., Honigberg B. M. Analysis of surface saccharides in Trichomonas vaginalis strains with various pathogenicity levels by fluorescein-conjugated plant lectins. Z Parasitenkd. 1983;69(2):149–159. doi: 10.1007/BF00926951. [DOI] [PubMed] [Google Scholar]