Abstract
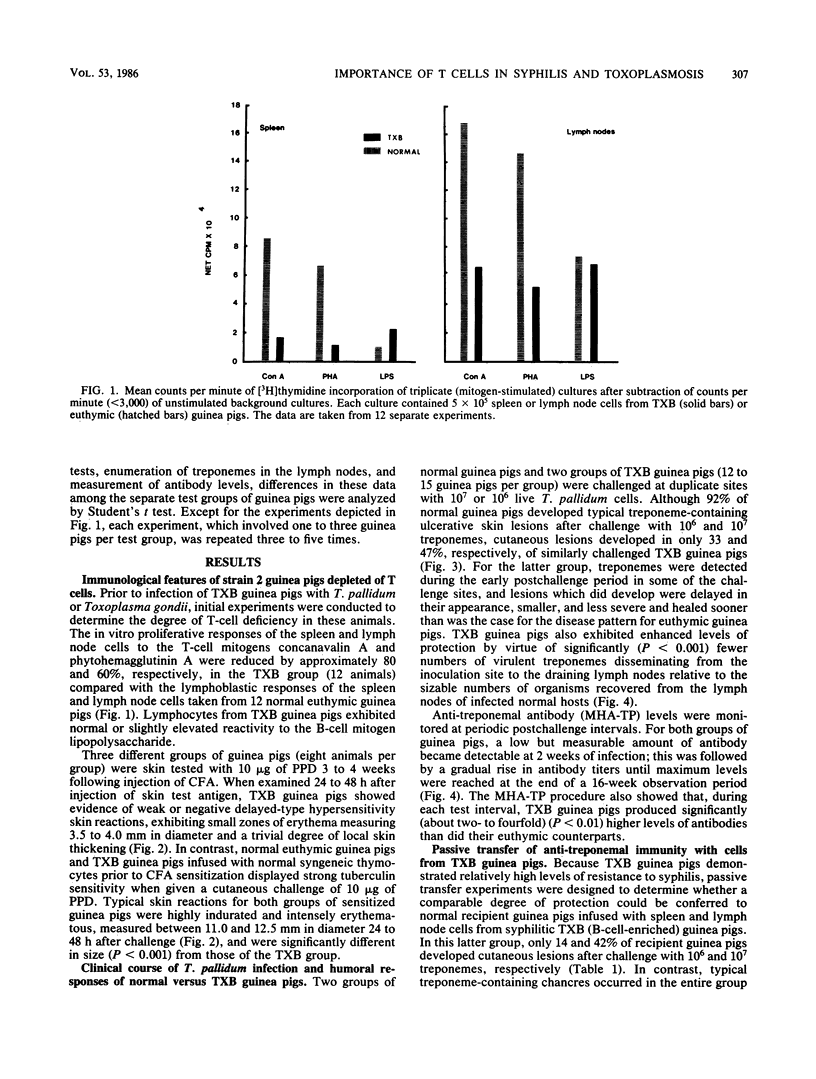
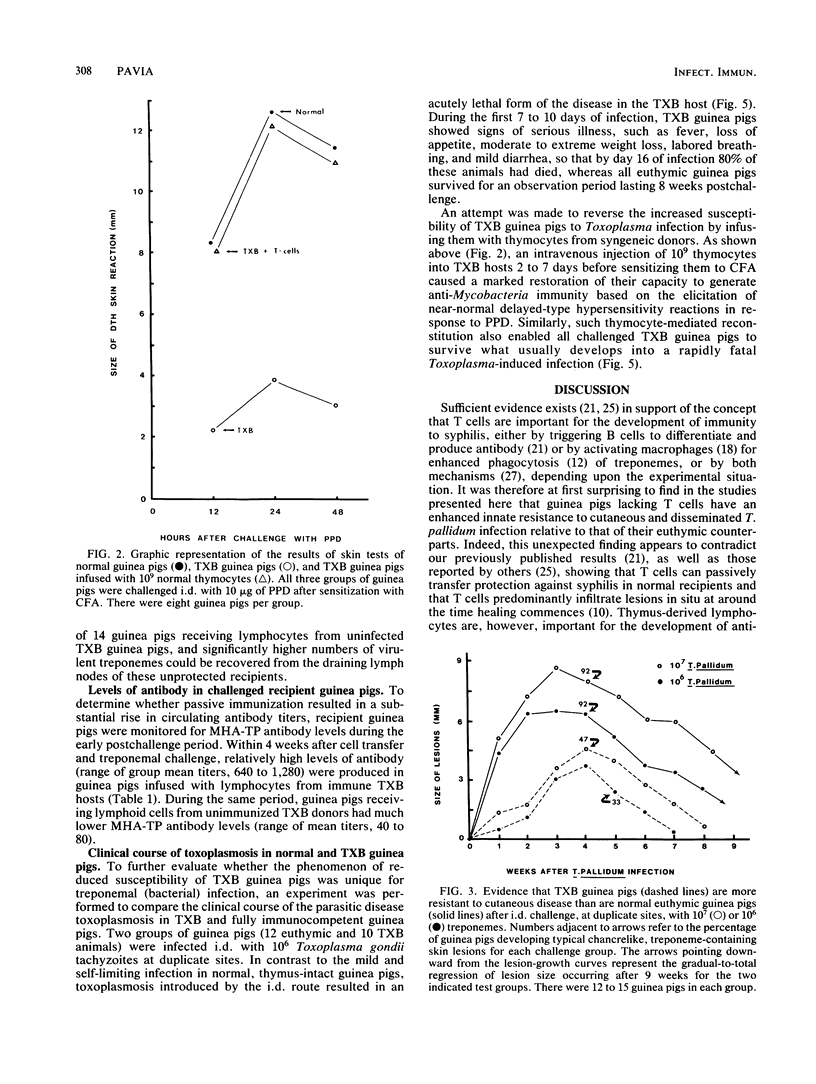
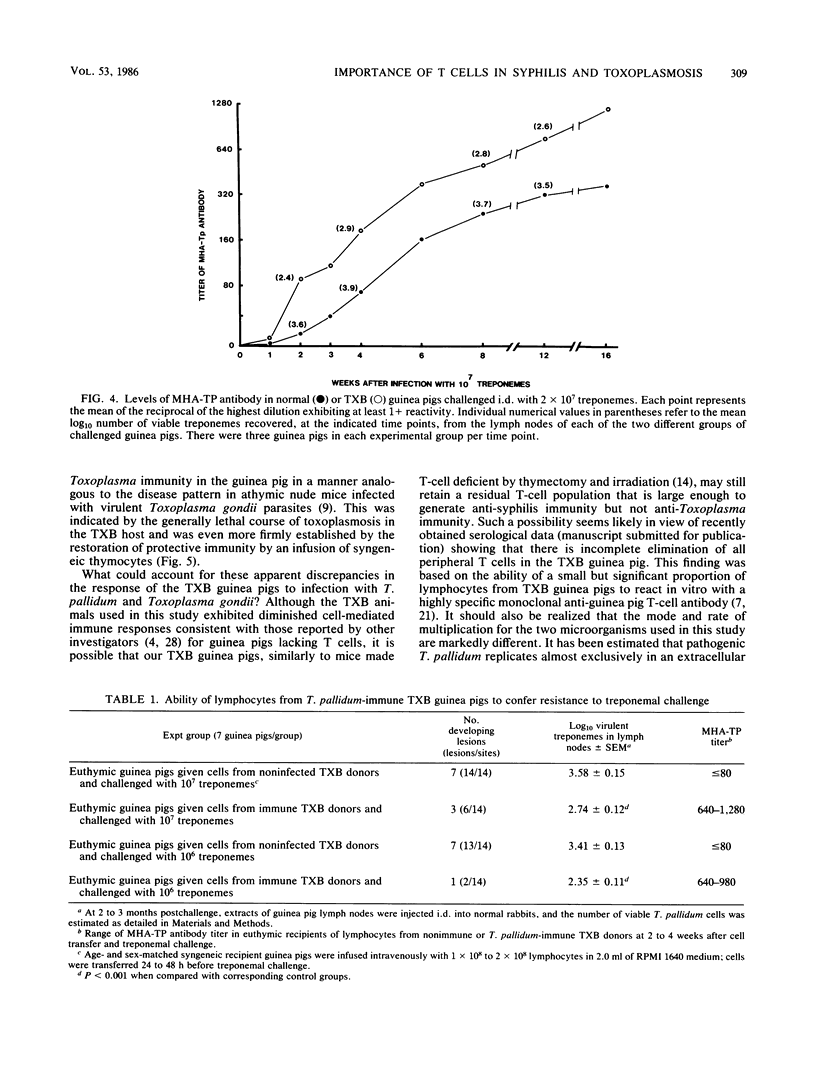
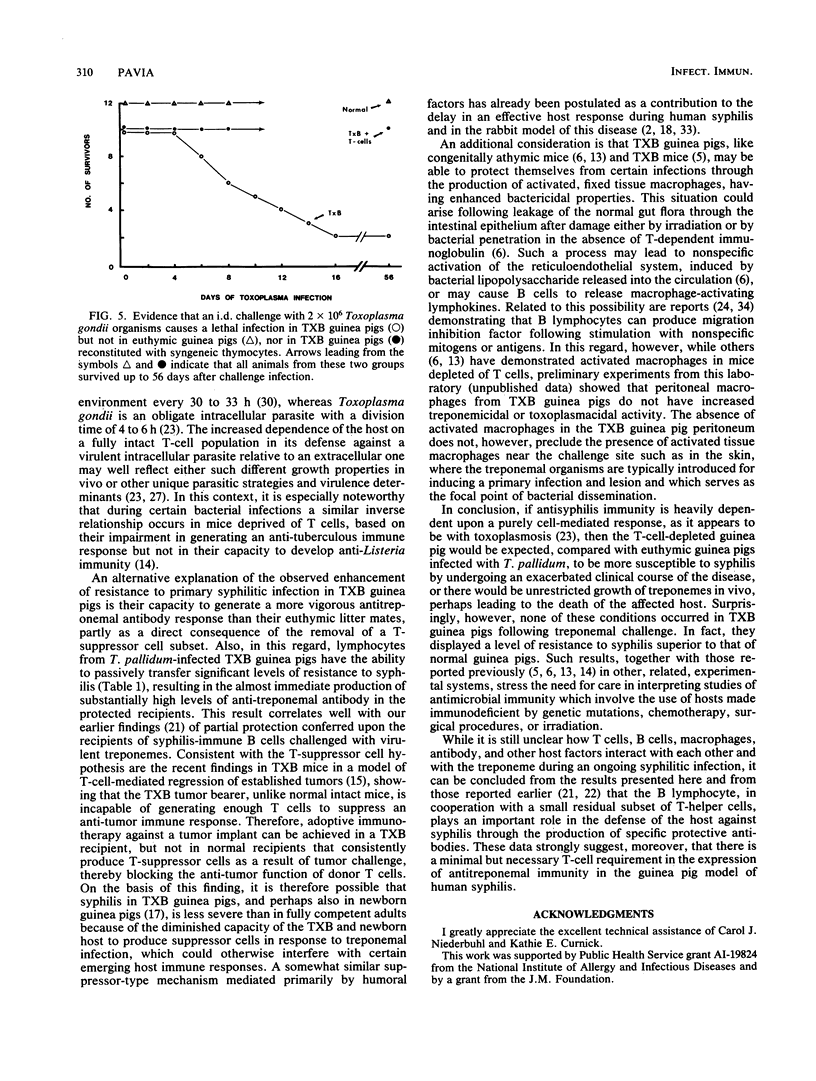
Strain 2 guinea pigs made T-cell deficient by thymectomy and irradiation and protected with syngeneic bone-marrow cells (TXB guinea pigs) have a surprisingly high level of resistance to cutaneous syphilis and to the dissemination of treponemes to the draining lymph node. Compared with normal euthymic controls infected with Treponema pallidum Nichols, syphilitic TXB guinea pigs developed fewer and less severe skin lesions and their lymph nodes contained lower numbers of treponemes. Associated with this evidence for enhanced innate resistance was the ability of the TXB host to produce, during each test interval of a primary infection, more antitreponemal antibodies than that of their euthymic counterparts. Similar levels of partial protection against cutaneous and disseminated syphilitic infection and elevated antibody levels occurred in challenged normal guinea pigs passively immunized with lymphocytes from T. pallidum-infected TXB donors. In contrast, the capacity of the TXB host to be protected against a lethal infection with the unrelated intracellular protozoan parasite Toxoplasma gondii was greatly impaired unless it received an intravenous infusion of normal syngeneic thymocytes. These seemingly paradoxical results are explained primarily in terms of a residual T-helper-cell population in the TXB guinea pig which is large and competent enough to generate antisyphilis, but not anti-Toxoplasma, immunity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azadegan A. A., Schell R. F., LeFrock J. L. Immune serum confers protection against syphilitic infection on hamsters. Infect Immun. 1983 Oct;42(1):42–47. doi: 10.1128/iai.42.1.42-47.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco D. R., Miller J. N., Hanff P. A. Humoral immunity in experimental syphilis: the demonstration of IgG as a treponemicidal factor in immune rabbit serum. J Immunol. 1984 Nov;133(5):2693–2697. [PubMed] [Google Scholar]
- Campos-Neto A., Levine H., Schlossman S. T. T cell regulation of specific B cell responses. J Immunol. 1978 Dec;121(6):2235–2240. [PubMed] [Google Scholar]
- Chan C., Kongshavn P. A., Skamene E. Enhanced primary resistance to Listeria monocytogenes in T cell-deprived mice. Immunology. 1977 Apr;32(4):529–537. [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Chiba J., Chused T. M., Leiserson W. M., Zweig S. E., Shevach E. M. Production and characterization of monoclonal antibodies to guinea pig lymphoid differentiation antigens. J Immunol Methods. 1983 Oct 14;63(2):247–261. doi: 10.1016/0022-1759(83)90429-5. [DOI] [PubMed] [Google Scholar]
- DeLAMATER E. D., SAURINO V. R., URBACH F. Studies on the immunology of spirochetoses. I. Effect of cortisone and experimental spirochetosis. Am J Syph Gonorrhea Vener Dis. 1952 Mar;36(2):127–139. [PubMed] [Google Scholar]
- Lindberg R. E., Frenkel J. K. Toxoplasmosis in nude mice. J Parasitol. 1977 Apr;63(2):219–221. [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Lloyd R. M., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol. 1980 Jan;124(1):461–467. [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Lloyd R. M., Sell S. Effect of cortisone administration on host-parasite relationships in early experimental syphilis. J Immunol. 1981 Oct;127(4):1361–1368. [PubMed] [Google Scholar]
- Lukehart S. A., Miller J. N. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol. 1978 Nov;121(5):2014–2024. [PubMed] [Google Scholar]
- Nickol A. D., Bonventre P. F. Anomalous high native resistance to athymic mice to bacterial pathogens. Infect Immun. 1977 Dec;18(3):636–645. doi: 10.1128/iai.18.3.636-645.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- North R. J. Models of adoptive T-cell-mediated regression of established tumors. Contemp Top Immunobiol. 1984;13:243–257. doi: 10.1007/978-1-4757-1445-6_12. [DOI] [PubMed] [Google Scholar]
- Pacha J., Metzger M., Smogór W., Michalska E., Podwińska J., Ruczkowska J. Effect of immunosuppressive agents on the course of experimental syphilis in rabbits. Arch Immunol Ther Exp (Warsz) 1979;27(1-2):45–51. [PubMed] [Google Scholar]
- Pavia C. S., Niederbuhl C. J. Acquired resistance and expression of a protective humoral immune response in guinea pigs infected with Treponema pallidum Nichols. Infect Immun. 1985 Oct;50(1):66–72. doi: 10.1128/iai.50.1.66-72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Niederbuhl C. J. Adoptive transfer of anti-syphilis immunity with lymphocytes from Treponema pallidum-infected guinea pigs. J Immunol. 1985 Oct;135(4):2829–2834. [PubMed] [Google Scholar]
- Pavia C. S., Niederbuhl C. J. Experimental infection of inbred guinea pigs with Treponema pallidum: development of lesions and formation of antibodies. Genitourin Med. 1985 Apr;61(2):75–81. doi: 10.1136/sti.61.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S. Transfer of resistance to syphilitic infection from maternal to newborn guinea pigs. Infect Immun. 1986 Jan;51(1):365–368. doi: 10.1128/iai.51.1.365-368.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavis C. S., Folds J. D., Baseman J. B. Cell-mediated immunity during syphilis. Br J Vener Dis. 1978 Jun;54(3):144–150. doi: 10.1136/sti.54.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklin R. E., MacDermott R. P., Chess L., Schlossman S. F., David J. R. Studies on mediator production by highly purified human T and B lymphocytes. J Exp Med. 1974 Nov 1;140(5):1303–1316. doi: 10.1084/jem.140.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell R. F., Chan J. K., LeFrock J. L., Bagasra O. Endemic syphilis: transfer of resistance to Treponema pallidum strain Bosnia A in hamsters with a cell suspension enriched in thymus-derived cells. J Infect Dis. 1980 Jun;141(6):752–758. doi: 10.1093/infdis/141.6.752. [DOI] [PubMed] [Google Scholar]
- Schell R. F., LeFrock J. L., Chan J. K. Transfer of resistance with syphilitic immune cells: lack of correlation with mitogenic activity. Infect Immun. 1982 Jan;35(1):187–192. doi: 10.1128/iai.35.1.187-192.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu S., Fonseca L. S., Hunter J. T., Rapp H. J. Mechanisms of immunological eradication of a syngeneic guinea pig tumor. II. Effect of methotrexate treatment and T cell depletion of the recipient on adoptive immunity. Transplantation. 1983 Jan;35(1):56–61. doi: 10.1097/00007890-198301000-00011. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Weiser R. S. Experimental syphilis in the rabbit: passive transfer of immunity with immunoglobulin G from immune serum. J Infect Dis. 1979 Dec;140(6):904–913. doi: 10.1093/infdis/140.6.904. [DOI] [PubMed] [Google Scholar]
- WICHER K., JAKUBOWSKI A. EFFECT OF CORTISONE ON THE COURSE OF EXPERIMENTAL SYPHILIS IN THE GUINEA-PIG. I. EFFECT OF PREVIOUSLY-ADMINISTERED CORTISONE ON GUINEA-PIGS INFECTED WITH TREPONEMA PALLIDUM INTRADERMALLY, INTRATESTICULARLY, AND INTRAVENOUSLY. Br J Vener Dis. 1964 Sep;40:213–216. doi: 10.1136/sti.40.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D. J., Grimble A. S. Why is the infectious stage of syphilis prolonged? Br J Vener Dis. 1974 Feb;50(1):45–49. doi: 10.1136/sti.50.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Sonozaki H., Cohen S. The production of migration inhibition factor by B and T cells of the guinea pig. J Exp Med. 1973 Oct 1;138(4):784–797. doi: 10.1084/jem.138.4.784. [DOI] [PMC free article] [PubMed] [Google Scholar]



