Abstract
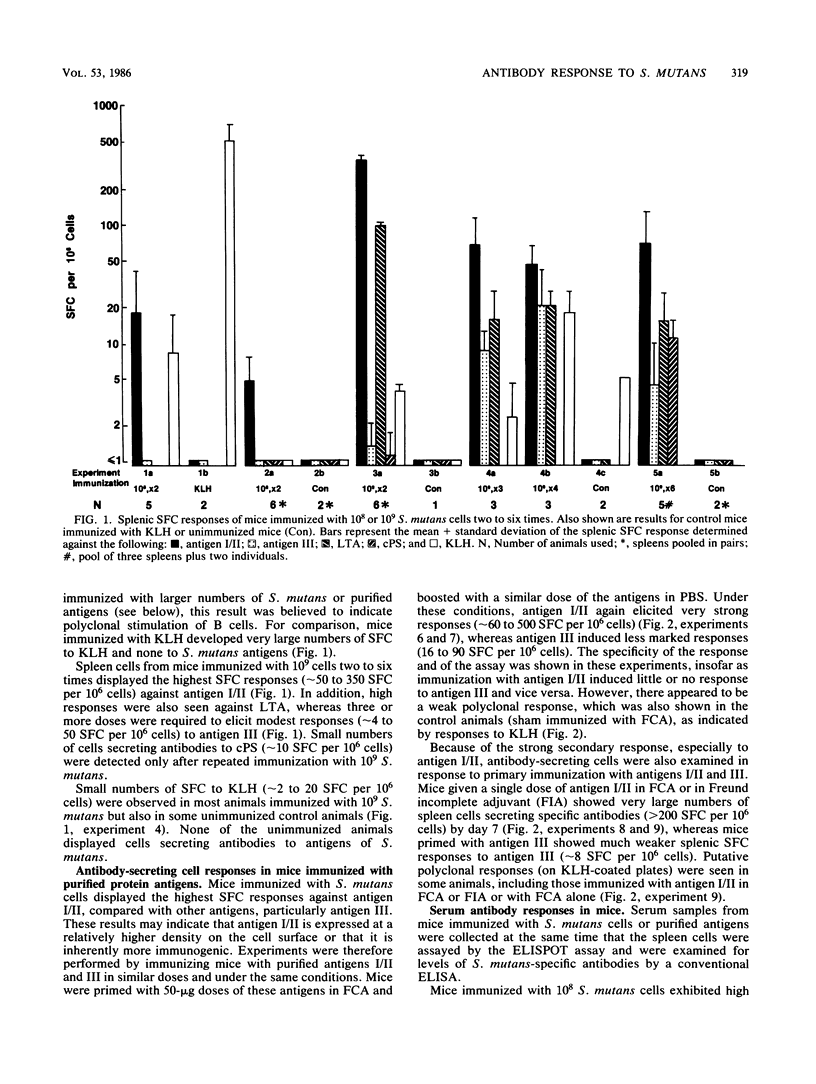
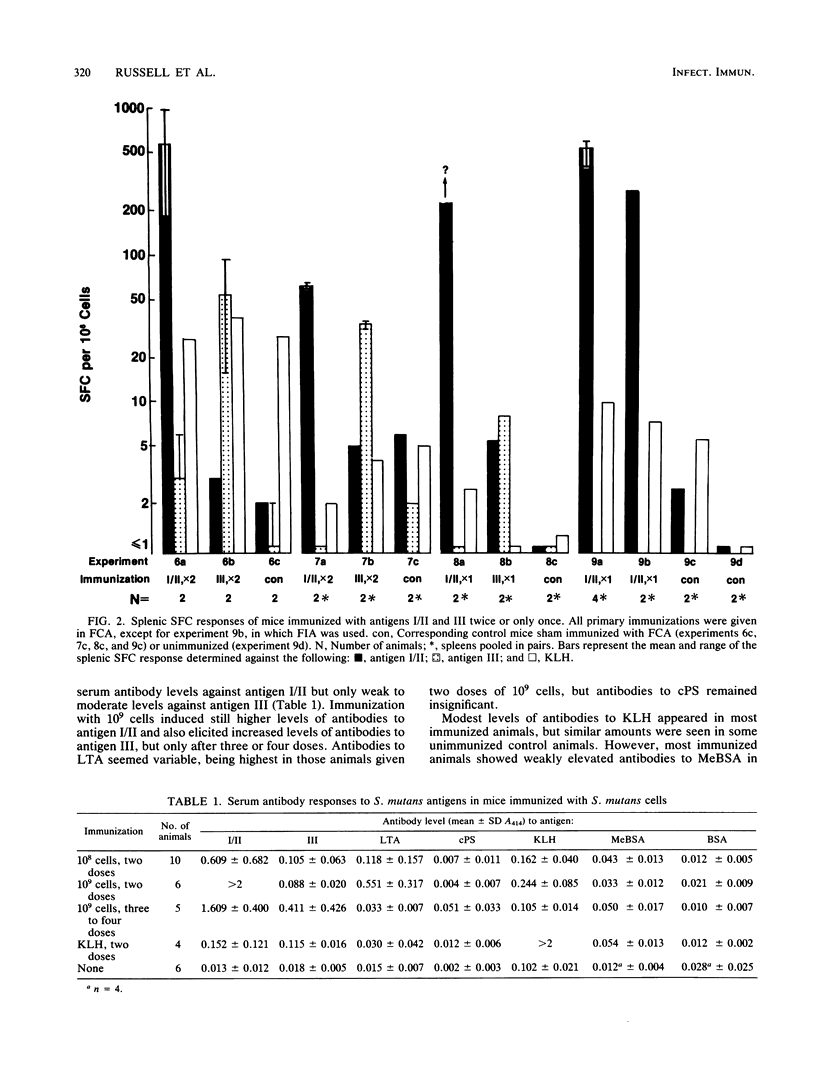
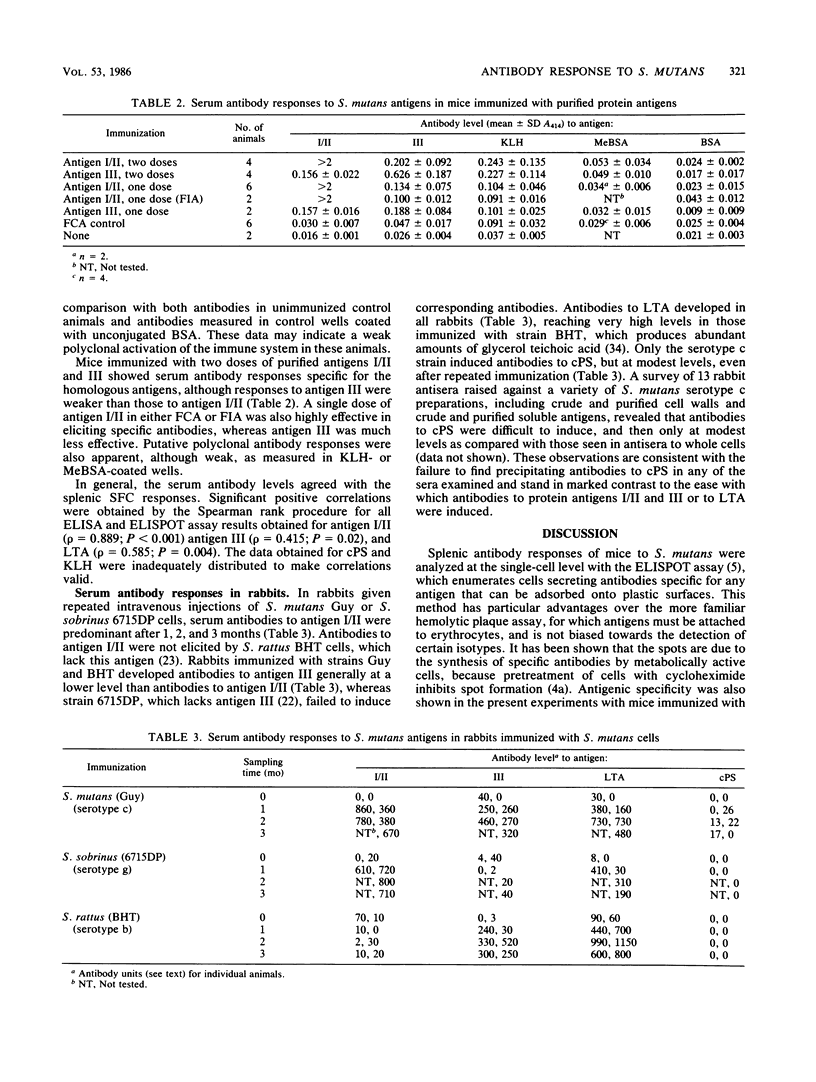
Immune responses of mice to Streptococcus mutans serotype c were analyzed by means of the enzyme-linked immunospot assay to determine the predominant specificities of the antibodies developed. In general, the numbers of splenic antibody-secreting cells correlated with serum antibody levels. A low dose (10(8) CFU) of killed whole cells injected twice intraperitoneally induced antibodies mainly against surface protein antigen I/II. A higher dose (10(9) CFU) given two to six times also resulted in a predominance of antigen I/II antibody-secreting cells and, in addition, antibody responses to surface protein antigen III and lipoteichoic acid occurred. Cells producing antibodies to serotype c polysaccharide were elicited only on repeated immunization. These results agreed with the development of antibodies in rabbits repeatedly immunized intravenously with killed whole cells of S. mutans, S. rattus, and S. sobrinus, which induced specific antibodies in accordance with the surface antigens that they express. Mice immunized twice with the same dose of purified antigens I/II and III developed greater numbers of antigen I/II splenic antibody-forming cells than antigen III splenic antibody-forming cells and higher serum antibody levels to antigen I/II than to antigen III. Furthermore, a single injection of antigen I/II but not of antigen III was sufficient to induce a strong specific-antibody response. Some evidence was also obtained for weak polyclonal stimulation of spleen cells by S. mutans cells and by antigen I/II, a result which could be relevant to the induction by S. mutans of antibodies reactive with mammalian tissues. It was concluded that for the antigens examined, S. mutans elicited the strongest antibody response against antigen I/II, which was also highly immunogenic in purified form.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beining P. R., Flannery G. M., Prescott B., Baker P. J. Influence of carrier-specific, thymus-derived cells on the immunologlobulin M antibody response to staphylococcal lipoteichoic acid. Infect Immun. 1980 Jul;29(1):132–139. doi: 10.1128/iai.29.1.132-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challacombe S. J., Bergmeier L. A., Czerkinsky C., Rees A. S. Natural antibodies in man to Streptococcus mutans: specificity and quantification. Immunology. 1984 May;52(1):143–150. [PMC free article] [PubMed] [Google Scholar]
- Chorpenning F. W., Cooper H. R., Rosen S. Cross-reactions of Streptococcus mutans due to cell wall teichoic acid. Infect Immun. 1975 Sep;12(3):586–591. doi: 10.1128/iai.12.3.586-591.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C. C., Nilsson L. A., Nygren H., Ouchterlony O., Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Dec 16;65(1-2):109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- Davis S. E., Munson P. J., Jaffe M. L., Rodbard D. Radioimmunoassay data processing with a small programmable calculator. J Immunoassay. 1980;1(1):15–25. doi: 10.1080/01971528008055773. [DOI] [PubMed] [Google Scholar]
- Dziarski R. Preferential induction of autoantibody secretion in polyclonal activation by peptidoglycan and lipopolysaccharide. I. In vitro studies. J Immunol. 1982 Mar;128(3):1018–1025. [PubMed] [Google Scholar]
- Fiedel B. A., Jackson R. W. Immunogenicity of a purified and carrier-complexed streptococcal lipoteichoic acid. Infect Immun. 1976 Jun;13(6):1585–1590. doi: 10.1128/iai.13.6.1585-1590.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forester H., Hunter N., Knox K. W. Characteristics of a high molecular weight extracellular protein of Streptococcus mutans. J Gen Microbiol. 1983 Sep;129(9):2779–2788. doi: 10.1099/00221287-129-9-2779. [DOI] [PubMed] [Google Scholar]
- Gambrell S. C., Jr, Allen J. M., Jr Stress concentrations at the apex of pinned, implanted teeth. J Dent Res. 1976 Jan-Feb;55(1):59–65. doi: 10.1177/00220345760550012501. [DOI] [PubMed] [Google Scholar]
- Holt R. G., Abiko Y., Saito S., Smorawinska M., Hansen J. B., Curtiss R., 3rd Streptococcus mutans genes that code for extracellular proteins in Escherichia coli K-12. Infect Immun. 1982 Oct;38(1):147–156. doi: 10.1128/iai.38.1.147-156.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling U., Westphal O. Synthesis and use of O-stearoyl polysaccharides in passive hemagglutination and hemolysis. Eur J Biochem. 1967 Mar;1(1):46–50. doi: 10.1007/978-3-662-25813-2_9. [DOI] [PubMed] [Google Scholar]
- Izui S., Eisenberg R. A., Dixon F. J. IgM rheumatoid factors in mice injected with bacterial lipopolysaccharides. J Immunol. 1979 May;122(5):2096–2102. [PubMed] [Google Scholar]
- Kutteh W. H., Koopman W. J., Conley M. E., Egan M. L., Mestecky J. Production of predominantly polymeric IgA by human peripheral blood lymphocytes stimulated in vitro with mitogens. J Exp Med. 1980 Nov 1;152(5):1424–1429. doi: 10.1084/jem.152.5.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner T., Russell M. W., Caldwell J., Smith R. Immunization with purified protein antigens from Streptococcus mutans against dental caries in rhesus monkeys. Infect Immun. 1981 Nov;34(2):407–415. doi: 10.1128/iai.34.2.407-415.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Song M., Krasse B., Olsson J. Biochemical and immunological differences between hydrophobic and hydrophilic strains of Streptococcus mutans. Infect Immun. 1984 Apr;44(1):68–75. doi: 10.1128/iai.44.1.68-75.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro I., Russell M. W. Ultrastructural localization of protein antigens I/II and III in Streptococcus mutans. Infect Immun. 1983 Jul;41(1):410–413. doi: 10.1128/iai.41.1.410-413.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popham A. M., Dresser D. W. Rheumatoid factors in mice: non-specific activators of heterophile rheumatoid factor production. Immunology. 1980 Nov;41(3):579–585. [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Bergmeier L. A., Zanders E. D., Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980 May;28(2):486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Challacombe S. J., Lehner T. Specificity of antibodies induced by Streptococcus mutans during immunization against dental caries. Immunology. 1980 May;40(1):97–106. [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Lehner T. Characterisation of antigens extracted from cells and culture fluids of Streptococcus mutans serotype c. Arch Oral Biol. 1978;23(1):7–15. doi: 10.1016/0003-9969(78)90047-x. [DOI] [PubMed] [Google Scholar]
- Russell M. W. Purification and properties of a protein surface antigen of Streptococcus mutants. Microbios. 1979;25(99):7–18. [PubMed] [Google Scholar]
- Russell M. W., Zanders E. D., Bergmeier L. A., Lehner T. Affinity purification and characterization of protease-susceptible antigen I of Streptococcus mutans. Infect Immun. 1980 Sep;29(3):999–1006. doi: 10.1128/iai.29.3.999-1006.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R., Beighton D., Cohen B. Immunisation of monkeys (Macaca fascicularis) with antigens purified from Streptococcus mutans. Br Dent J. 1982 Feb 2;152(3):81–84. doi: 10.1038/sj.bdj.4804751. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol. 1979 Sep;114(1):109–115. doi: 10.1099/00221287-114-1-109. [DOI] [PubMed] [Google Scholar]
- Schöller M., Klein J. P., Sommer P., Frank R. Common antigens of streptococcal and nonstreptococcal oral bacteria: characterization of wall-associated protein and comparison with extracellular protein antigen. Infect Immun. 1983 Jun;40(3):1186–1191. doi: 10.1128/iai.40.3.1186-1191.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Hsu K. C., Gotschlich E. C. Electron microscopic studies on streptococci. I. M antigen. J Exp Med. 1969 Nov 1;130(5):1063–1091. doi: 10.1084/jem.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M., McGhee J. R., Koopman W. J., Hamada S., Michalek S. M. Lymphoid cell responses to bacterial cell wall components: polyclonal and immune responses of murine B cells to Streptococcus mutans carbohydrate antigens. J Immunol. 1981 Nov;127(5):2106–2112. [PubMed] [Google Scholar]
- Wicken A. J., Evans J. D., Campbell L. K., Knox K. W. Teichoic acids from chemostat-grown cultures of Streptococcus mutans and Lactobacillus plantarum. Infect Immun. 1982 Oct;38(1):1–7. doi: 10.1128/iai.38.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Gibbens J. W., Knox K. W. Comparative studies on the isolation of membrane lipoteichoic acid from Lactobacillus fermenti. J Bacteriol. 1973 Jan;113(1):365–372. doi: 10.1128/jb.113.1.365-372.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]



