Abstract
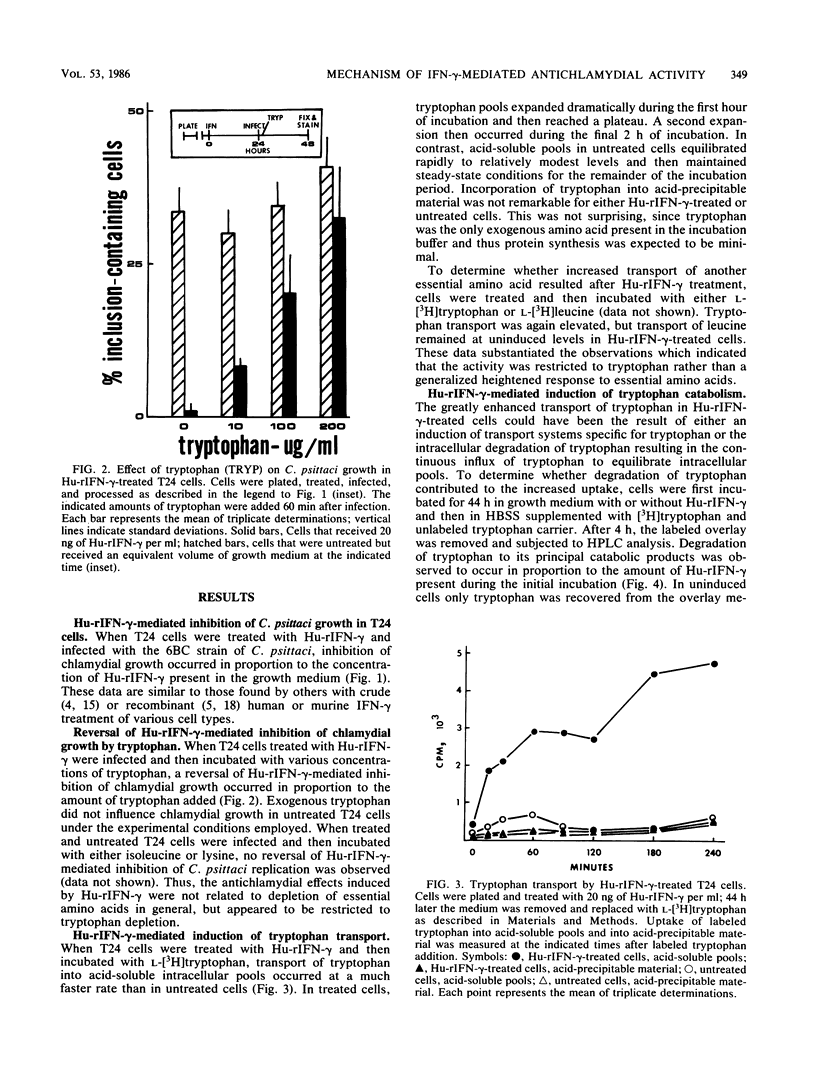
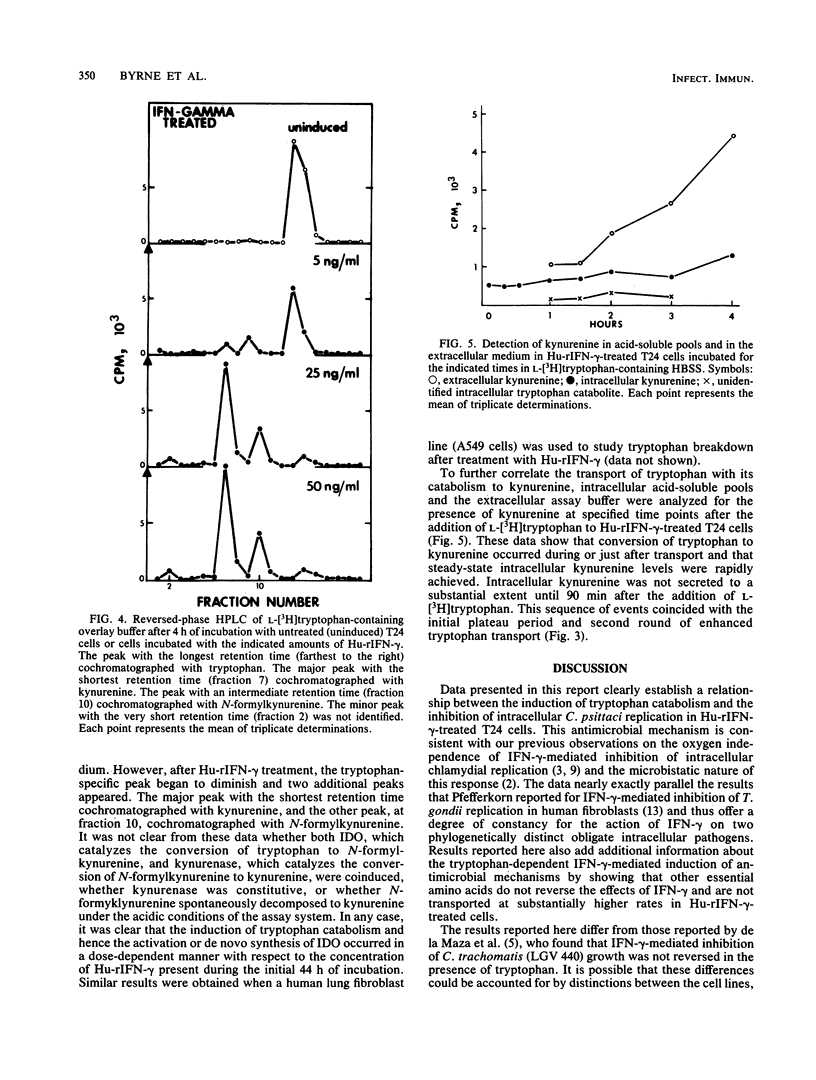
Human uroepithelial (T24) cells were incubated for 24 h in the presence of various concentrations of human recombinant gamma interferon (Hu-rIFN-gamma) and then infected with the 6BC strain of Chlamydia psittaci. This resulted in a reduction of intracellular chlamydial inclusion development in proportion to the concentration of Hu-rIFN-gamma present when Giemsa-stained cells were examined by light microscopy 24 h after infection. When tryptophan was added to Hu-rIFN-gamma-treated cells just after infection, reversal of the Hu-rIFN-gamma-mediated inhibition occurred in proportion to the concentration of tryptophan added. Addition of either isoleucine or lysine did not result in reversal of the antichlamydial state. Transport of L-[3H]tryptophan into acid-soluble intracellular pools was found to be greatly enhanced in Hu-rIFN-gamma-treated T24 cells compared with the rates measured for untreated cells. Transport of [3H]leucine was not increased in treated cells. Cells treated with Hu-rIFN-gamma also degraded L-[3H]tryptophan to catabolites that cochromatographed with N-formylkynurenine and kynurenine as measured by high-performance liquid chromatography. We conclude that Hu-rIFN-gamma-mediated inhibition of intracellular C. psittaci replication in T24 cells occurs by depletion of the essential amino acid tryptophan, most likely via the induction of indoleamine-2,3-dioxygenase, the initial enzyme of tryptophan catabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brummer E., Morrison C. J., Stevens D. A. Recombinant and natural gamma-interferon activation of macrophages in vitro: different dose requirements for induction of killing activity against phagocytizable and nonphagocytizable fungi. Infect Immun. 1985 Sep;49(3):724–730. doi: 10.1128/iai.49.3.724-730.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Faubion C. L. Inhibition of Chlamydia psittaci in oxidatively active thioglycolate-elicited macrophages: distinction between lymphokine-mediated oxygen-dependent and oxygen-independent macrophage activation. Infect Immun. 1983 May;40(2):464–471. doi: 10.1128/iai.40.2.464-471.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Faubion C. L. Lymphokine-mediated microbistatic mechanisms restrict Chlamydia psittaci growth in macrophages. J Immunol. 1982 Jan;128(1):469–474. [PubMed] [Google Scholar]
- Byrne G. I., Krueger D. A. Lymphokine-mediated inhibition of Chlamydia replication in mouse fibroblasts is neutralized by anti-gamma interferon immunoglobulin. Infect Immun. 1983 Dec;42(3):1152–1158. doi: 10.1128/iai.42.3.1152-1158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaishi O. Indoleamine 2,3-dioxygenase--with special reference to the mechanism of interferon action. Biken J. 1985 Jun;28(1-2):39–49. [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Activated human monocytes inhibit the intracellular multiplication of Legionnaires' disease bacteria. J Exp Med. 1981 Nov 1;154(5):1618–1635. doi: 10.1084/jem.154.5.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Byrne G. I., Rothermel C. D., Cartelli D. M. Lymphokine enhances oxygen-independent activity against intracellular pathogens. J Exp Med. 1983 Jul 1;158(1):234–239. doi: 10.1084/jem.158.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med. 1980 Dec 1;152(6):1596–1609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacy C. A., James S. L., Benjamin W. R., Farrar J. J., Hockmeyer W. T., Meltzer M. S. Activation of macrophages for microbicidal and tumoricidal effector functions by soluble factors from EL-4, a continuous T cell line. Infect Immun. 1983 May;40(2):820–824. doi: 10.1128/iai.40.2.820-824.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Guyre P. M. Inhibition of growth of Toxoplasma gondii in cultured fibroblasts by human recombinant gamma interferon. Infect Immun. 1984 May;44(2):211–216. doi: 10.1128/iai.44.2.211-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984 Feb;81(3):908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Murray H. W. Gamma-interferon is the factor in lymphokine that activates human macrophages to inhibit intracellular Chlamydia psittaci replication. J Immunol. 1983 Nov;131(5):2542–2544. [PubMed] [Google Scholar]
- Sasada M., Johnston R. B., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980 Jul 1;152(1):85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Kleinschmidt W. J. Functional identity between murine gamma interferon and macrophage activating factor. Nature. 1983 Sep 15;305(5931):239–240. doi: 10.1038/305239a0. [DOI] [PubMed] [Google Scholar]
- Shemer Y., Sarov I. Inhibition of growth of Chlamydia trachomatis by human gamma interferon. Infect Immun. 1985 May;48(2):592–596. doi: 10.1128/iai.48.2.592-596.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedersky L. P., Benton C. V., Berger W. H., Rinderknecht E., Harkins R. N., Palladino M. A. Biological and antigenic similarities of murine interferon-gamma and macrophage-activating factor. J Exp Med. 1984 Mar 1;159(3):812–827. doi: 10.1084/jem.159.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Thompson H. A., Winkler H. H. Interferon-gamma inhibits growth of Coxiella burnetii in mouse fibroblasts. Infect Immun. 1984 Sep;45(3):781–783. doi: 10.1128/iai.45.3.781-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Cloned mouse interferon-gamma inhibits the growth of Rickettsia prowazekii in cultured mouse fibroblasts. J Exp Med. 1983 Dec 1;158(6):2159–2164. doi: 10.1084/jem.158.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong S., Lau S. Rapid separation of tryptophan, kynurenines, and indoles using reversed-phase high-performance liquid chromatography. J Chromatogr. 1979 Jul 13;175(2):343–346. doi: 10.1016/s0021-9673(00)89443-1. [DOI] [PubMed] [Google Scholar]
- Yoshida R., Imanishi J., Oku T., Kishida T., Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc Natl Acad Sci U S A. 1981 Jan;78(1):129–132. doi: 10.1073/pnas.78.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Maza L. M., Peterson E. M., Fennie C. W., Czarniecki C. W. The anti-chlamydial and anti-proliferative activities of recombinant murine interferon-gamma are not dependent on tryptophan concentrations. J Immunol. 1985 Dec;135(6):4198–4200. [PubMed] [Google Scholar]


