Abstract
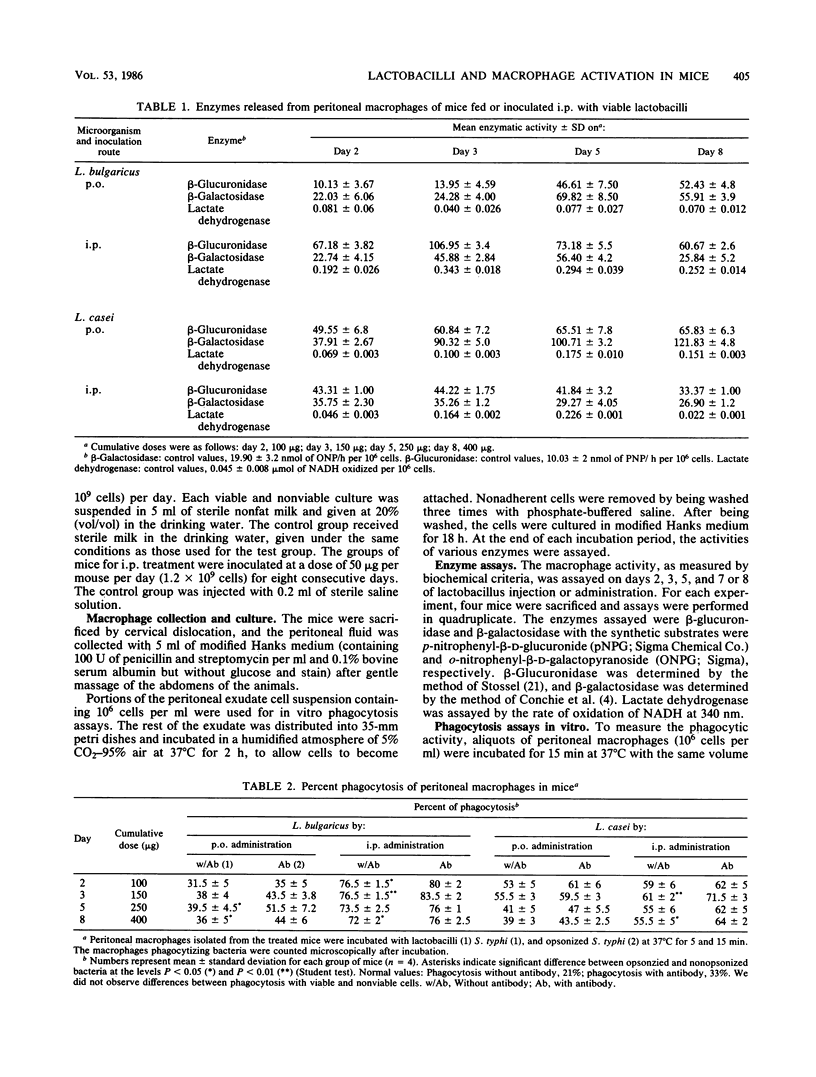
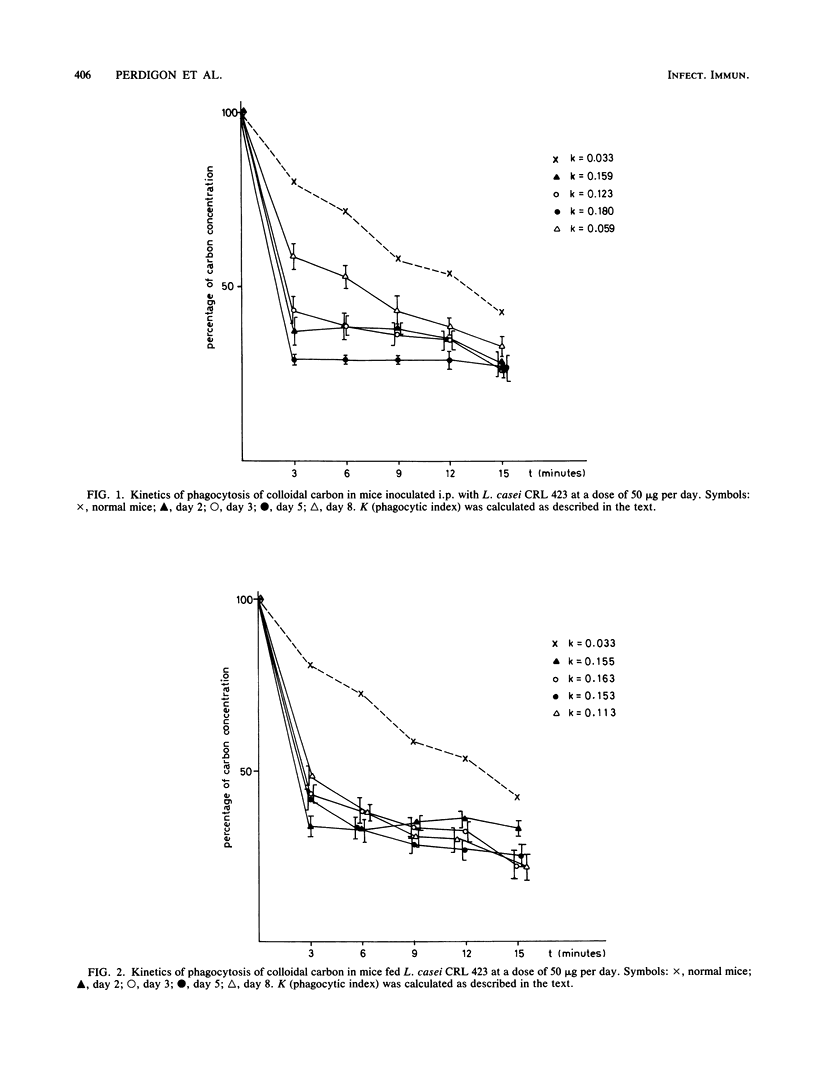
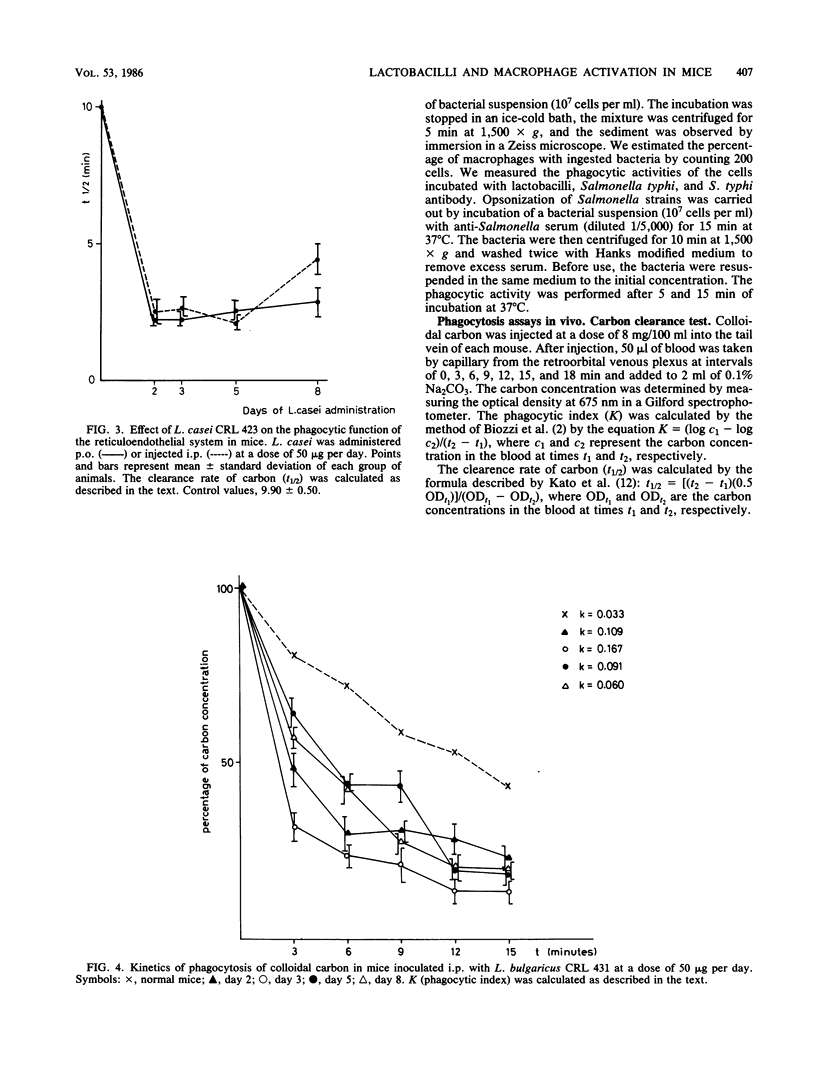
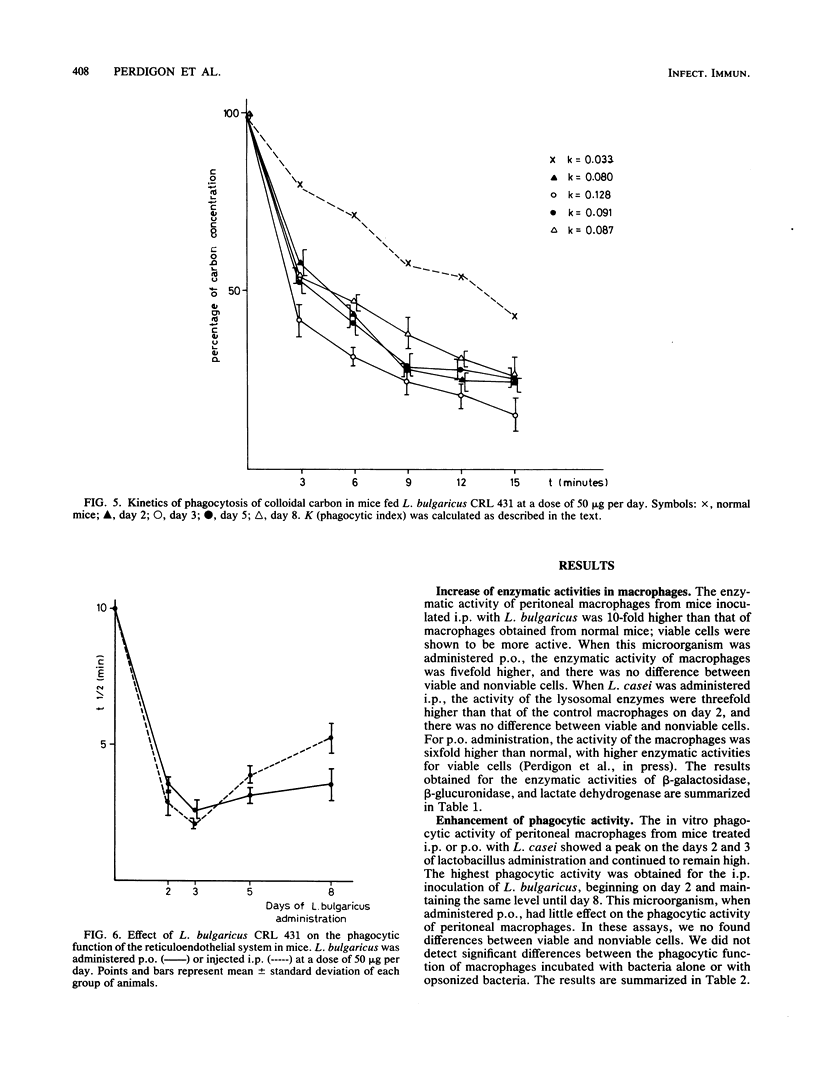
The effect of perorally (p.o) administered Lactobacillus casei and L. bulgaricus on macrophage activation in mice was studied. L. casei and L. bulgaricus were administered p.o. to mice for 8 days. The macrophage activation was measured on days 2, 3, 5, and 8 of lactobacillus administration by using biochemical and functional criteria. We measured the release of lysosomal hydrolases, the level of a nonlysosomal enzyme, and in vitro phagocytic activity of mouse peritoneal macrophages. All the assays were performed comparatively with mice inoculated with L. casei and L. bulgaricus (viable and nonviable cells) intraperitoneally (i.p.) at the same dose as for p.o. administration. The phagocytic activity was significantly higher in mice treated i.p. than in control mice. For p.o. administration, there was an increase only when L. casei was used. L. bulgaricus had little effect. No differences were found between viable and nonviable cells. The phagocytic function of the reticuloendothelial system was tested by the carbon clearance test, which showed that L. casei and L. bulgaricus accelerate the phagocytic function in mice treated p.o and i.p., from day 2 onward. These observations show that L. casei and L. bulgaricus given by p.o. administration are able to activate macrophages in mice and suggest that these bacteria, when passing through the intestinal tract, may be responsible for the enhanced host immune response. This fact is very significant because the diet includes fermented and manufactured products containing lactobacilli.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer H., Paronetto F., Burns W. A., Einheber A. The enhancing effect of the microbial flora on macrophage function and the immune response. A study in germfree mice. J Exp Med. 1966 Jun 1;123(6):1013–1024. doi: 10.1084/jem.123.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloksma N., de Heer E., van Dijk H., Willers J. M. Adjuvanticity of lactobacilli. I. Differential effects of viable and killed bacteria. Clin Exp Immunol. 1979 Aug;37(2):367–375. [PMC free article] [PubMed] [Google Scholar]
- CONCHIE J., FINDLAY J., LEVVY G. A. Mammalian glycosidases; distribution in the body. Biochem J. 1959 Feb;71(2):318–325. doi: 10.1042/bj0710318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemsa D., Kubelka C., Debatin K. M., Krammer P. H. Activation of macrophages by lymphokines from T-cell clones: evidence for different macrophage-activating factors. Mol Immunol. 1984 Dec;21(12):1267–1276. doi: 10.1016/0161-5890(84)90020-8. [DOI] [PubMed] [Google Scholar]
- Goodenough E. R., Kleyn D. H. Influence of viable yogurt microflora on digestion of lactose by the rat. J Dairy Sci. 1976 Apr;59(4):601–606. doi: 10.3168/jds.S0022-0302(76)84247-6. [DOI] [PubMed] [Google Scholar]
- Iannello D., Bonina L., Delfino D., Berlinghieri M. C., Gismondo M. R., Mastroeni P. Effect of oral administration of a variety of bacteria on depressed macrophage functions in tumour-bearing rats. Ann Immunol (Paris) 1984 May-Jun;135C(3):345–352. doi: 10.1016/s0769-2625(84)80964-2. [DOI] [PubMed] [Google Scholar]
- Kato I., Kobayashi S., Yokokura T., Mutai M. Antitumor activity of Lactobacillus casei in mice. Gan. 1981 Aug;72(4):517–523. [PubMed] [Google Scholar]
- Kato I., Yokokura T., Mutai M. Augmentation of mouse natural killer cell activity by Lactobacillus casei and its surface antigens. Microbiol Immunol. 1984;28(2):209–217. doi: 10.1111/j.1348-0421.1984.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Kato I., Yokokura T., Mutai M. Macrophage activation by Lactobacillus casei in mice. Microbiol Immunol. 1983;27(7):611–618. doi: 10.1111/j.1348-0421.1983.tb00622.x. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T., Carter P. B. Requirement for a bacterial flora before mice generate cells capable of mediating the delayed hypersensitivity reaction to sheep red blood cells. J Immunol. 1979 Jun;122(6):2624–2629. [PubMed] [Google Scholar]
- Namba Y., Hidaka Y., Taki K., Morimoto T. Effect of oral administration of lysozyme or digested bacterial cell walls on immunostimulation in guinea pigs. Infect Immun. 1981 Feb;31(2):580–583. doi: 10.1128/iai.31.2.580-583.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder J., Baggiolini M. Secretion of lysosomal hydrolases by stimulated and nonstimulated macrophages. J Exp Med. 1978 Aug 1;148(2):435–450. doi: 10.1084/jem.148.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]


