Abstract
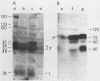
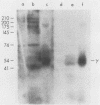
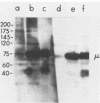
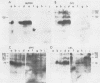
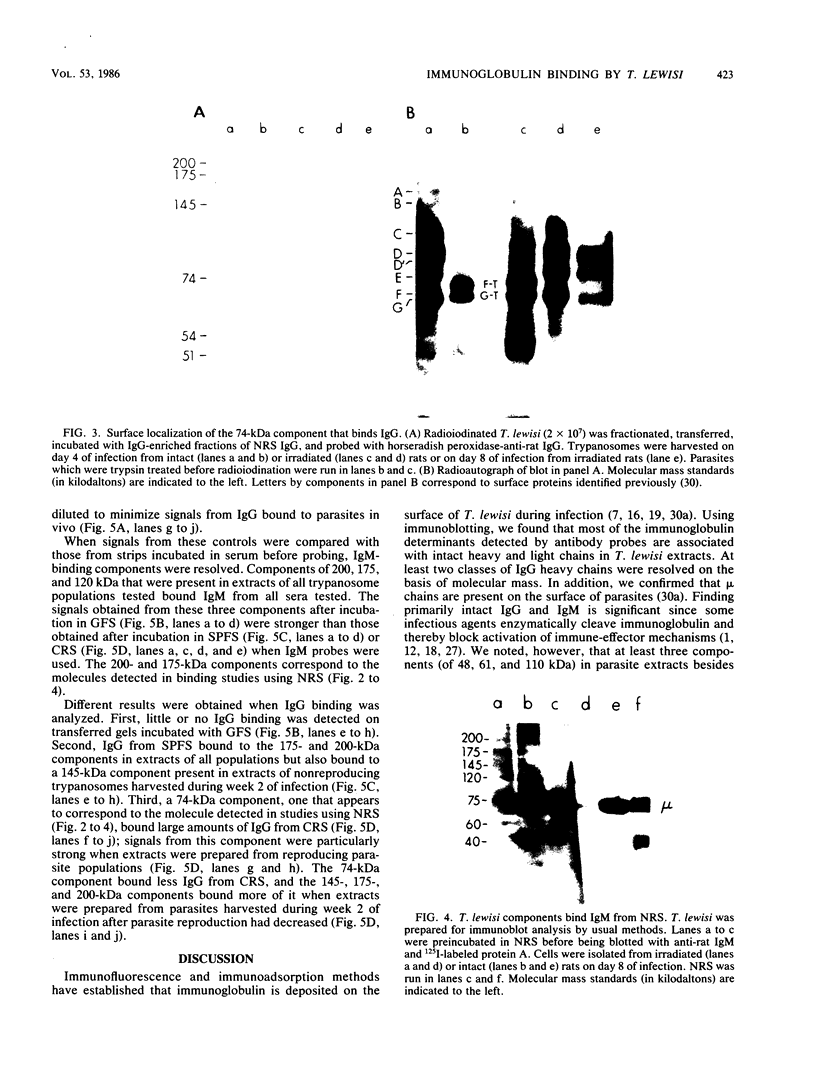
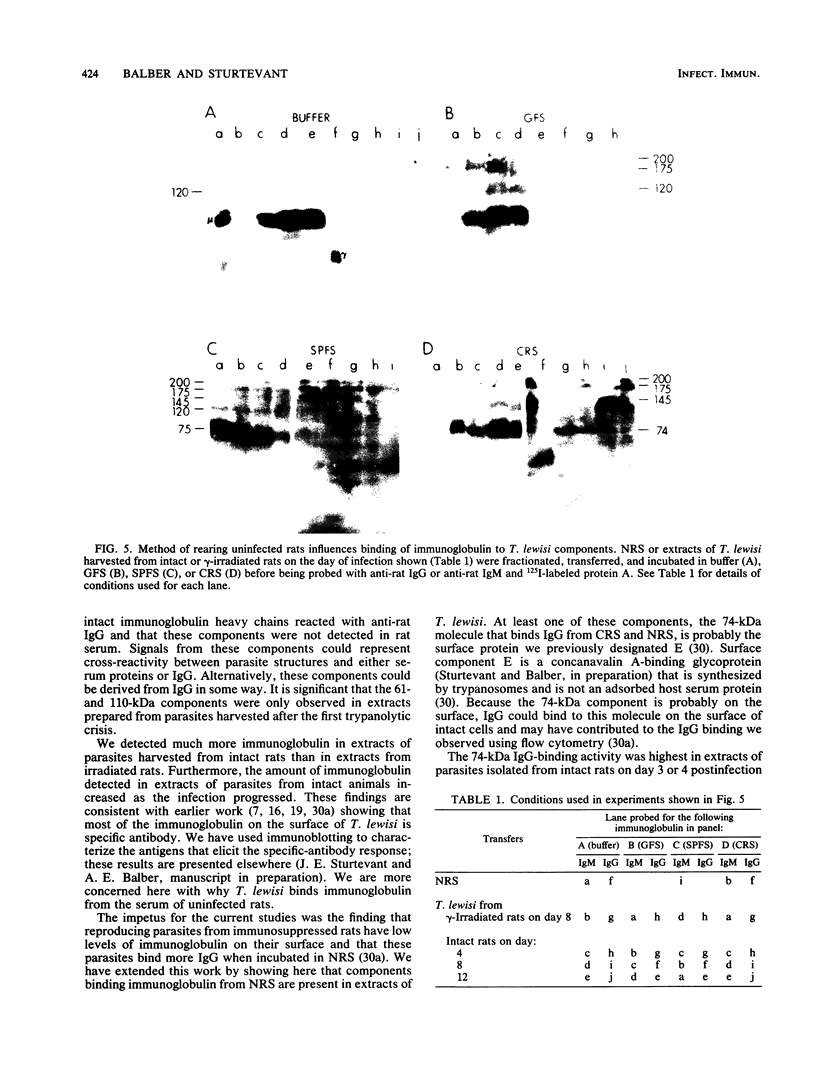
Detergent extracts of whole Trypanosoma lewisi were fractionated by polyacrylamide gel electrophoresis and transferred to nitrocellulose for immunoblotting analysis. Antibody probes to rat immunoglobulin G (IgG) and IgM detected intact gamma chains, mu chains, and light chains in extracts. The amount of immunoglobulin detected increased as the infection progressed. Transfers were also incubated in serum from conventionally reared (CRS), specific-pathogen-free (SPFS), or germ-free rats before being probed with anti-rat IgG or anti-rat IgM. Components of 200, 175, and 120 kilodaltons (kDa) bound IgM from all sera tested and were present in extracts of trypanosomes isolated from lethally irradiated or intact rats on different days during infection. No parasite components bound IgG from serum of germ-free rats. However, 145-, 175-, and 200-kDa components bound IgG from CRS and SPFS. A 74-kDa protein was the major IgG-binding component in extracts of reproducing parasites. This component bound much more IgG from CRS than it bound from SPFS. The 74-kDa protein was removed from parasites by mild trypsinization and corresponded to a major surface glycoprotein detected when intact cells were radioiodinated. These results indicate that natural antibodies to T. lewisi exist in rats or that these parasites have surface proteins that bind immunoglobulins without regard to antigenic specificity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auriault C., Ouaissi M. A., Torpier G., Eisen H., Capron A. Proteolytic cleavage of IgG bound to the Fc receptor of Schistosoma mansoni schistosomula. Parasite Immunol. 1981 Spring;3(1):33–44. doi: 10.1111/j.1365-3024.1981.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Bogucki M. S., Seed J. R. Parasite-bound heterospecific antibody in experimental African trypanosomiasis. J Reticuloendothel Soc. 1978 Feb;23(2):89–101. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clarkson A. B., Jr, Mellow G. H. Rheumatoid factor-like immunoglobulin M protects previously uninfected rat pups and dams from Trypanosoma lewisi. Science. 1981 Oct 9;214(4517):186–188. doi: 10.1126/science.7025211. [DOI] [PubMed] [Google Scholar]
- D'Alesandro P. A., Clarkson A. B., Jr Trypanosoma lewisi: avidity and adsorbability of ablastin, the rat antibody inhibiting parasite reproduction. Exp Parasitol. 1980 Dec;50(3):384–396. doi: 10.1016/0014-4894(80)90041-7. [DOI] [PubMed] [Google Scholar]
- D'Alesandro P. A. The relation of agglutinins to antigenic variation of Trypanosoma lewisi. J Protozool. 1976 May;23(2):256–261. doi: 10.1111/j.1550-7408.1976.tb03766.x. [DOI] [PubMed] [Google Scholar]
- De Miranda-Santos I. K., Campos-Neto A. Receptor for immunoglobulin Fc on pathogenic but not on nonpathogenic protozoa of the Trypanosomatidae. J Exp Med. 1981 Dec 1;154(6):1732–1742. doi: 10.1084/jem.154.6.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley P. Comparative immunological analysis of host plasma proteins bound to bloodstream forms of Trypanosoma brucei subspecies. Infect Immun. 1978 Aug;21(2):605–612. doi: 10.1128/iai.21.2.605-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew P. A., Jenkin C. R. Metabolic activity of Trypanosoma lewisi cultured in vitro in the presence of normal or ablastinic rat serum. Comp Biochem Physiol B. 1985;80(4):889–894. doi: 10.1016/0305-0491(85)90479-1. [DOI] [PubMed] [Google Scholar]
- Dwyer D. M. Immunologic and fine structure evidence of avidly bound host serum proteins in the surface coat of a bloodstream trypanosome. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1222–1226. doi: 10.1073/pnas.73.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen H., Tallan I. Tetrahymena pyriformis recovers from antibody immobilisation by producing univalent antibody fragments. Nature. 1977 Dec 8;270(5637):514–515. doi: 10.1038/270514a0. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Allison A. C. Natural agglutinins to African trypanosomes. Parasite Immunol. 1983 Nov;5(6):539–546. doi: 10.1111/j.1365-3024.1983.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Jenkin C. R. Surface immunoglobulins, a possible mechanism for the persistence of Trypanosoma lewisi in the circulation of rats. Aust J Exp Biol Med Sci. 1977 Jun;55(3):275–280. doi: 10.1038/icb.1977.22. [DOI] [PubMed] [Google Scholar]
- Ferrante A. The role of natural agglutinins and trypanolytic activity in host specificity of Trypanosoma musculi. Parasite Immunol. 1984 Nov;6(6):509–517. doi: 10.1111/j.1365-3024.1984.tb00821.x. [DOI] [PubMed] [Google Scholar]
- Fick R. B., Jr, Baltimore R. S., Squier S. U., Reynolds H. Y. IgG proteolytic activity of Pseudomonas aeruginosa in cystic fibrosis. J Infect Dis. 1985 Apr;151(4):589–598. doi: 10.1093/infdis/151.4.589. [DOI] [PubMed] [Google Scholar]
- Giannini S. H., D'Alesandro P. A. Isolation of protective antigens from Trypanosoma lewisi by using trypanostatic (ablastic) immunoglobulin G from the surface coat. Infect Immun. 1984 Feb;43(2):617–621. doi: 10.1128/iai.43.2.617-621.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini S. H., D'Alesandro P. A. Trypanosoma lewisi: accumulation of antigen-specific host IgG as a component of the surface coat during the course of infection in the rat. Exp Parasitol. 1979 Jun;47(3):342–355. doi: 10.1016/0014-4894(79)90087-0. [DOI] [PubMed] [Google Scholar]
- Giannini S. H., D'Alesandro P. A. Trypanostatic activity of rat IgG purified from the surface coat of Trypanosoma lewisi. J Parasitol. 1982 Oct;68(5):765–773. [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Langone J. J. Protein A of Staphylococcus aureus and related immunoglobulin receptors produced by streptococci and pneumonococci. Adv Immunol. 1982;32:157–252. [PubMed] [Google Scholar]
- Medgyesi G. A., Füst G., Gergely J., Bazin H. Classes and subclasses of rat immunoglobulins: interaction with the complement system and with staphylococcal protein A. Immunochemistry. 1978 Feb;15(2):125–129. doi: 10.1016/0161-5890(78)90052-4. [DOI] [PubMed] [Google Scholar]
- Patton C. L. The ablastin phenomenon: inhibition of membrane function. Exp Parasitol. 1975 Dec;38(3):357–369. doi: 10.1016/0014-4894(75)90122-8. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Wistar R., Jr Loss of antibody activity in human immunoglobulin A exposed extracellular immunoglobulin A proteases of Neisseria gonorrhoeae and Streptococcus sanguis. Infect Immun. 1977 Jul;17(1):130–135. doi: 10.1128/iai.17.1.130-135.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux J., Picque M. T., Bazin H., Biserte G. Rat IgG subclasses: differences in affinity to protein A-sepharose. Mol Immunol. 1981 Jul;18(7):639–645. doi: 10.1016/0161-5890(81)90035-3. [DOI] [PubMed] [Google Scholar]
- Schraw W. P., Vaughan G. L. Trypanosoma lewisi: alterations in membrane function in the rat. Exp Parasitol. 1979 Aug;48(1):15–26. doi: 10.1016/0014-4894(79)90050-x. [DOI] [PubMed] [Google Scholar]
- Sturtevant J. E., Balber A. E. Externally disposed membrane polypeptides of intact and protease-treated Trypanosoma lewisi correlated with sensitivity to alternate complement pathway-mediated lysis. Infect Immun. 1983 Dec;42(3):869–875. doi: 10.1128/iai.42.3.869-875.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant J. E., Balber A. E. Flow cytometric analysis of immunoglobulin and complement component C3 on the surface of Trypanosoma lewisi. J Protozool. 1986 May;33(2):197–203. doi: 10.1111/j.1550-7408.1986.tb05589.x. [DOI] [PubMed] [Google Scholar]
- Torpier G., Capron A., Ouaissi M. A. Receptor for IgG(Fc) and human beta2-microglobulin on S. mansoni schistosomula. Nature. 1979 Mar 29;278(5703):447–449. doi: 10.1038/278447a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C., Fleit H., Mellman I. S. Structural Aspects and Heterogeneity of Immunoglobulin Fc Receptors. Adv Immunol. 1981;31:247–270. doi: 10.1016/s0065-2776(08)60922-0. [DOI] [PubMed] [Google Scholar]
- Widders P. R., Stokes C. R., Newby T. J., Bourne F. J. Nonimmune binding of equine immunoglobulin by the causative organism of contagious equine metritis, Taylorella equigenitalis. Infect Immun. 1985 May;48(2):417–421. doi: 10.1128/iai.48.2.417-421.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gee A. L., Rovis L. Trypanosoma vivax: absence of host protein on the surface coat. Exp Parasitol. 1981 Feb;51(1):124–132. doi: 10.1016/0014-4894(81)90049-7. [DOI] [PubMed] [Google Scholar]