Abstract
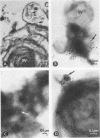
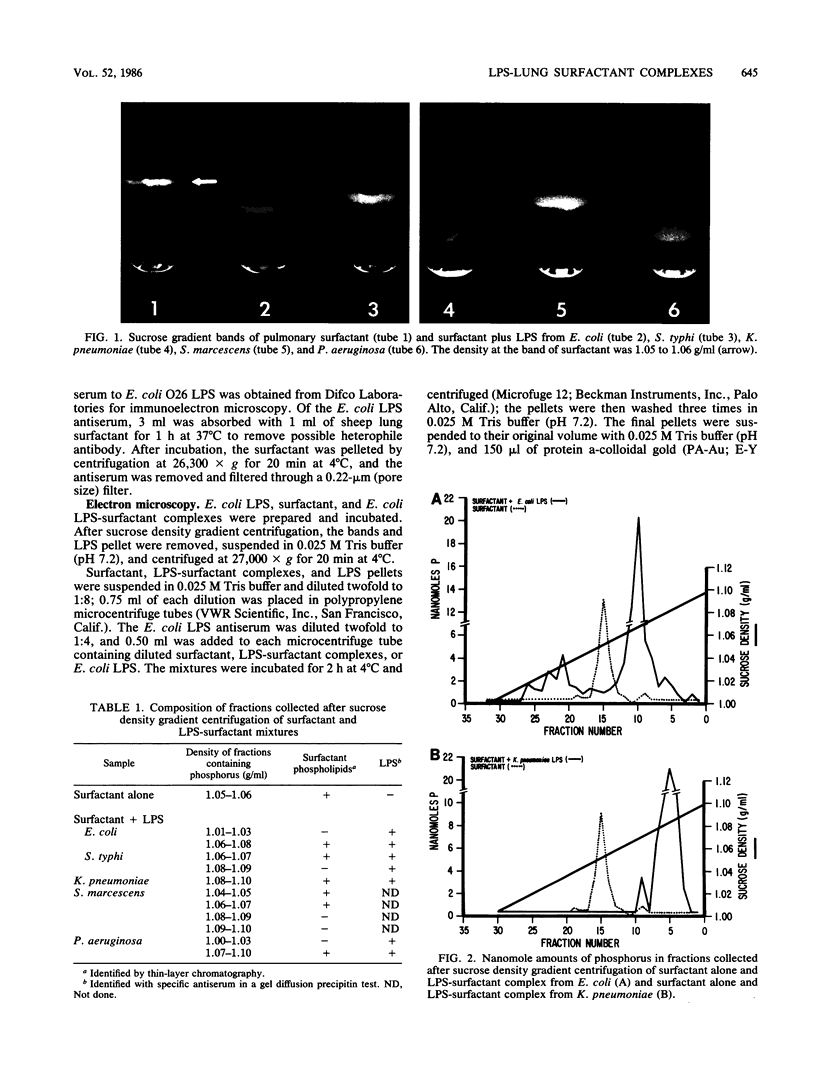
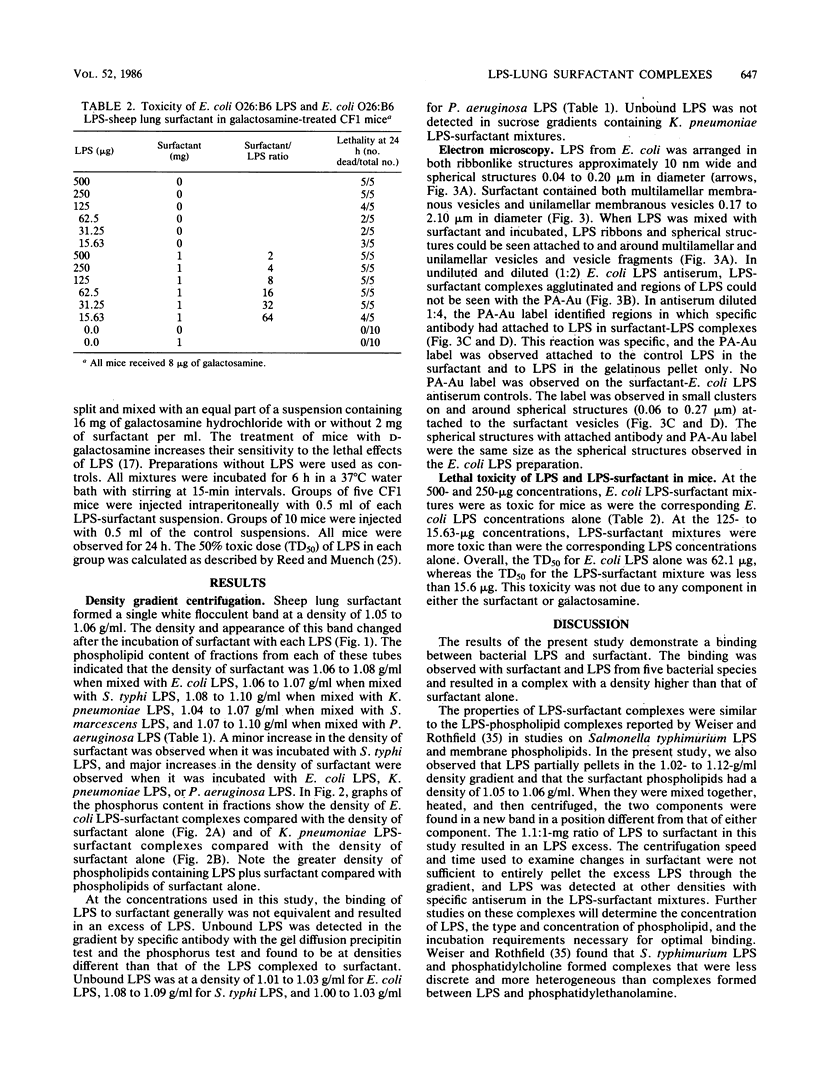
Lipopolysaccharides (LPS) from Escherichia coli, Salmonella typhi, Klebsiella pneumoniae, Serratia marcescens, or Pseudomonas aeruginosa were mixed with pulmonary surfactant to investigate their in vitro interaction. After 6 h of incubation at 37 degrees C, LPS-surfactant mixtures were examined by sucrose density gradient centrifugation. The E. coli LPS-surfactant mixture was examined by immunoelectron microscopy with protein A-colloidal gold. The binding that occurred between LPS and the surfactant vesicles resulted in a complex with a density higher than the density of the surfactant alone. The protein A-colloidal gold identified LPS in the LPS-surfactant complexes. The toxicity of E. coli LPS was enhanced by complexing with the surfactant when compared with the intraperitoneal injection into CF1 mice, even at a 64:1 ratio of surfactant to LPS. The complexing of LPS and surfactant in the lung may alter the physiologic properties of surfactant that contribute to the physiopathological changes observed with some types of pneumonia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert R. K., Lakshminarayan S., Hildebrandt J., Kirk W., Butler J. Increased surface tension favors pulmonary edema formation in anesthetized dogs' lungs. J Clin Invest. 1979 May;63(5):1015–1018. doi: 10.1172/JCI109369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano K., Fukushi K. Chemical and ultrastructural comparison of endotoxins extracted from Salmonella minnesota wild type and R mutants. Microbiol Immunol. 1984;28(2):149–159. doi: 10.1111/j.1348-0421.1984.tb00666.x. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baughman R. P., Stein E., MacGee J., Rashkin M., Sahebjami H. Changes in fatty acids in phospholipids of the bronchoalveolar fluid in bacterial pneumonia and in adult respiratory distress syndrome. Clin Chem. 1984 Apr;30(4):521–523. [PubMed] [Google Scholar]
- Benedetto D. A., Shands J. W., Jr, Shah D. O. The interaction of bacterial lipopolysaccharide with phospholipid bilayers and monolayers. Biochim Biophys Acta. 1973 Mar 16;298(2):145–157. doi: 10.1016/0005-2736(73)90346-5. [DOI] [PubMed] [Google Scholar]
- Bessa S. M., Dalmasso A. P., Goodale R. L., Jr Studies on the mechanism of endotoxin-induced increase of alveolocapillary premeability. Proc Soc Exp Biol Med. 1974 Dec;147(3):701–705. doi: 10.3181/00379727-147-38421. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brogden K. A., Cutlip R. C., Lehmkuhl H. D. Response of sheep after localized deposition of lipopolysaccharide in the lung. Exp Lung Res. 1984;7(2):123–132. doi: 10.3109/01902148409069673. [DOI] [PubMed] [Google Scholar]
- Brogden K. A., Rebers P. A. Serologic examination of the Westphal-type lipopolysaccharides of Pasteurella multocida. Am J Vet Res. 1978 Oct;39(10):1680–1682. [PubMed] [Google Scholar]
- Brogden K. A., Rimler R. B. Lysates of turkey-grown Pasteurella multocida: examination of vaccine preparations by electron microscopy. Am J Vet Res. 1982 Feb;43(2):304–309. [PubMed] [Google Scholar]
- Chalvardjian A., Rudnicki E. Determination of lipid phosphorus in the nanomolar range. Anal Biochem. 1970 Jul;36(1):225–226. doi: 10.1016/0003-2697(70)90352-0. [DOI] [PubMed] [Google Scholar]
- Davies M., Stewart-Tull D. E., Jackson D. M. The binding of lipopolysaccharide from Escherichia coli to mammalian cell membranes and its effect on liposomes. Biochim Biophys Acta. 1978 Apr 4;508(2):260–276. doi: 10.1016/0005-2736(78)90329-2. [DOI] [PubMed] [Google Scholar]
- Davies M., Stewart-Tull D. E. The affinity of bacterial polysaccharide-containing fractions for mammalian cell membranes and its relationship to immunopotentiating activity. Biochim Biophys Acta. 1981 Apr 22;643(1):17–29. doi: 10.1016/0005-2736(81)90215-7. [DOI] [PubMed] [Google Scholar]
- Davis W. B., Barsoum I. S., Ramwell P. W., Yeager H., Jr Human alveolar macrophages: effects of endotoxin in vitro. Infect Immun. 1980 Dec;30(3):753–758. doi: 10.1128/iai.30.3.753-758.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Bøg-Hansen T. C., Back U., Galanos C. Interaction of lipopolysaccharides with plasma high-density lipoprotein in rats. Infect Immun. 1980 May;28(2):373–380. doi: 10.1128/iai.28.2.373-380.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groniowski J. A. Fine structural basis of pulmonary surfactant. Int Rev Exp Pathol. 1983;25:183–238. [PubMed] [Google Scholar]
- Harrison L. H., Beller J. J., Hinshaw L. B., Coalson J. J., Greenfield L. J. Effects of endotoxin on pulmonary capillary permeability, ultrastructure, and surfactant. Surg Gynecol Obstet. 1969 Oct;129(4):723–733. [PubMed] [Google Scholar]
- Henry J. N. The effect of shock on pulmonary alveolar surfactant. Its role in refractory respiratory insufficiency of the critically ill or severely injured patient. J Trauma. 1968 Sep;8(5):756–773. [PubMed] [Google Scholar]
- Jobe A., Ikegami M., Glatz T., Yoshida Y., Diakomanolis E., Padbury J. Duration and characteristics of treatment of premature lambs with natural surfactant. J Clin Invest. 1981 Feb;67(2):370–375. doi: 10.1172/JCI110044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Magoon M. W., Wright J. R., Baritussio A., Williams M. C., Goerke J., Benson B. J., Hamilton R. L., Clements J. A. Subfractionation of lung surfactant. Implications for metabolism and surface activity. Biochim Biophys Acta. 1983 Jan 7;750(1):18–31. doi: 10.1016/0005-2760(83)90200-x. [DOI] [PubMed] [Google Scholar]
- Marks M. I., Ziegler E. J., Douglas H., Corbeil L. B., Braude A. I. Induction of immunity against lethal Haemophilus influenzae type b infection by Escherichia coli core lipopolysaccharide. J Clin Invest. 1982 Apr;69(4):742–749. doi: 10.1172/JCI110512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Horne R. W. Reassociation of purified lipopolysaccharide and phospholipid of the bacterial cell envelope: electron microscopic and monolayer studies. J Bacteriol. 1967 May;93(5):1705–1721. doi: 10.1128/jb.93.5.1705-1721.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTNICK A. I., SOLOFF L. A. ATELECTASIS WITH PNEUMONIA: A PATHOPHYSIOLOGIC STUDY. Ann Intern Med. 1964 Jan;60:39–46. doi: 10.7326/0003-4819-60-1-39. [DOI] [PubMed] [Google Scholar]
- Shands J. W., Jr, Graham J. A., Nath K. The morphologic structure of isolated bacterial lipopolysaccharide. J Mol Biol. 1967 Apr 14;25(1):15–21. doi: 10.1016/0022-2836(67)90275-6. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Nikaido H. Physical interaction between lipid A and phospholipids: a study with spin-labeled phospholipids. Rev Infect Dis. 1984 Jul-Aug;6(4):488–492. doi: 10.1093/clinids/6.4.488. [DOI] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R. The modification of biophysical and endotoxic properties of bacterial lipopolysaccharides by serum. J Clin Invest. 1978 Dec;62(6):1313–1324. doi: 10.1172/JCI109252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M. M., Rothfield L. The reassociation of lipopolysaccharide, phospholipid, and transferase enzymes of the bacterial cell envelope. Isolation of binary and ternary complexes. J Biol Chem. 1968 Mar 25;243(6):1320–1328. [PubMed] [Google Scholar]