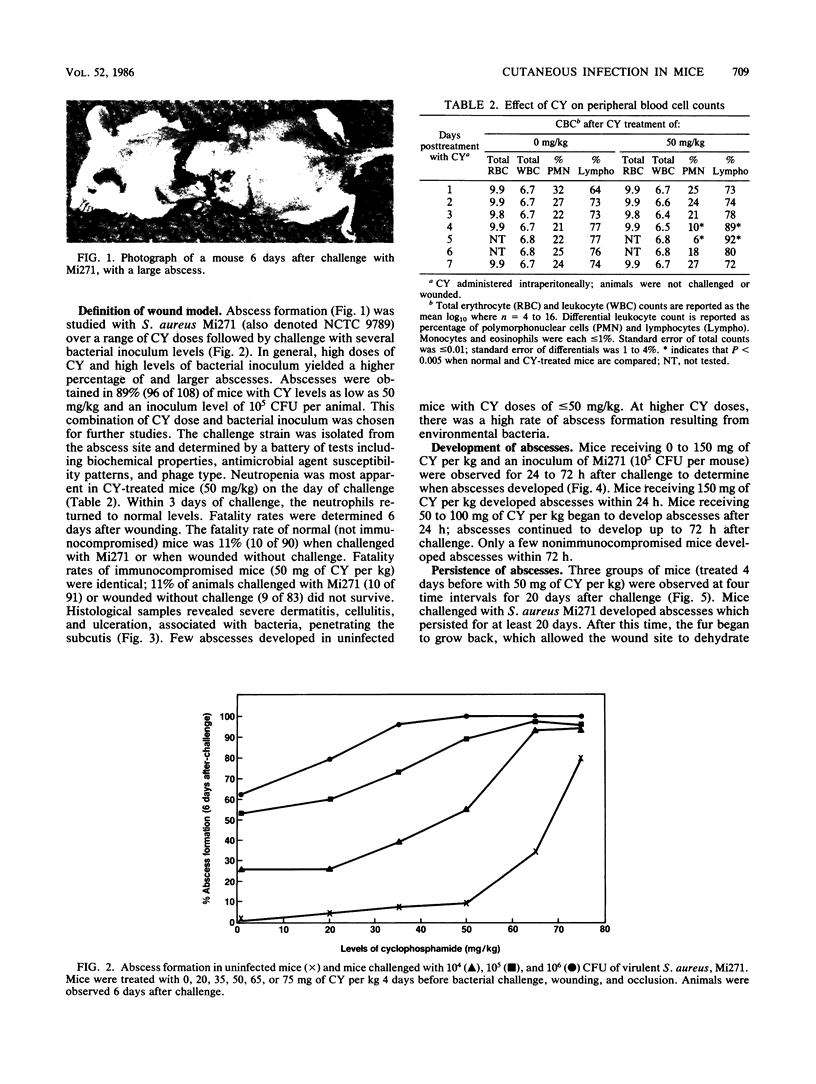
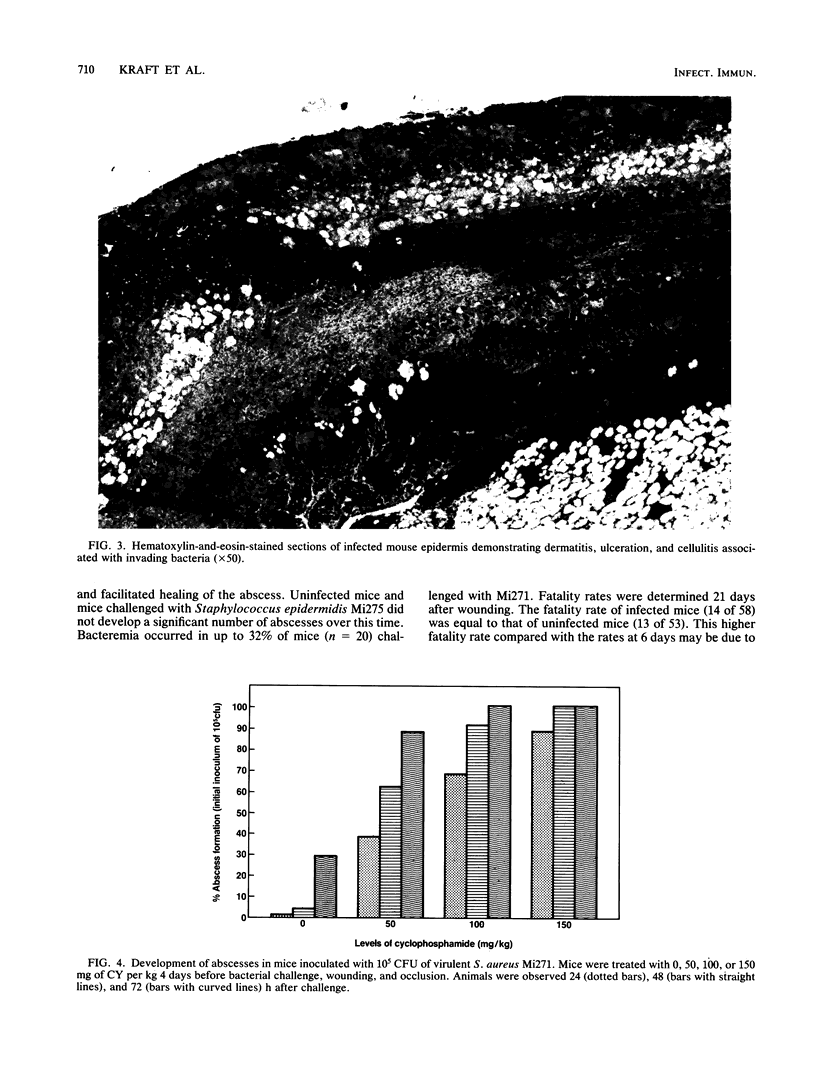
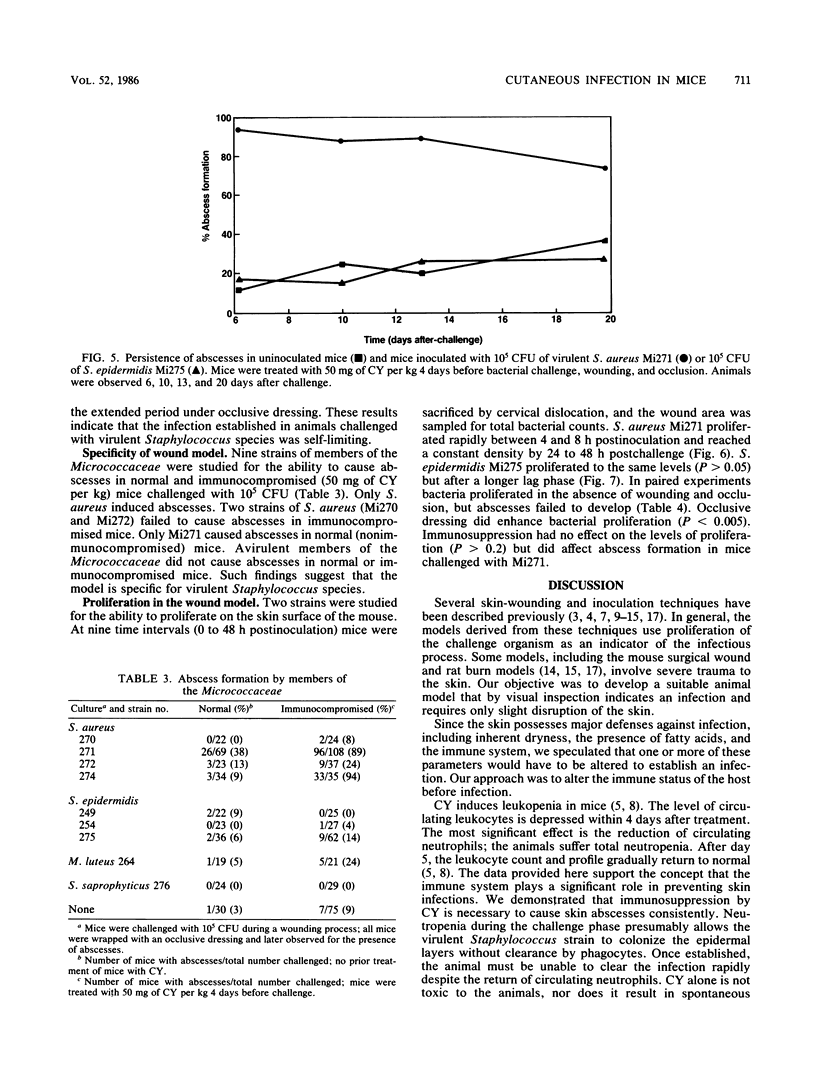
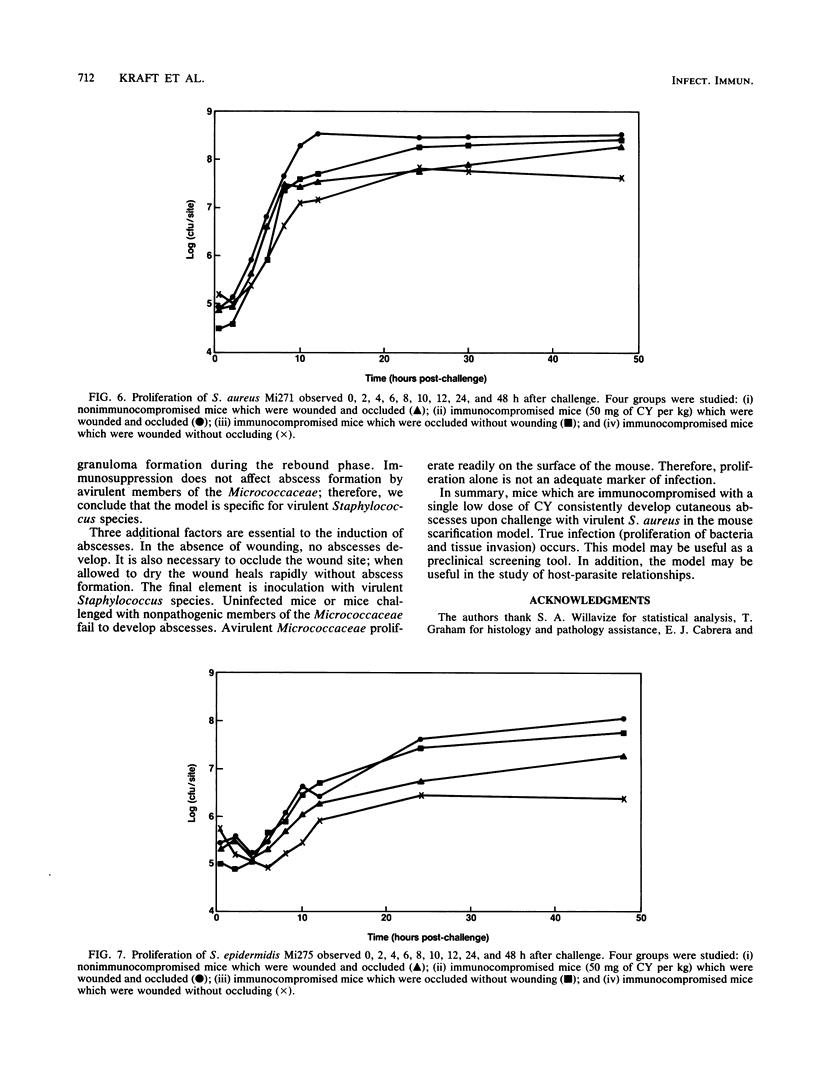
Abstract
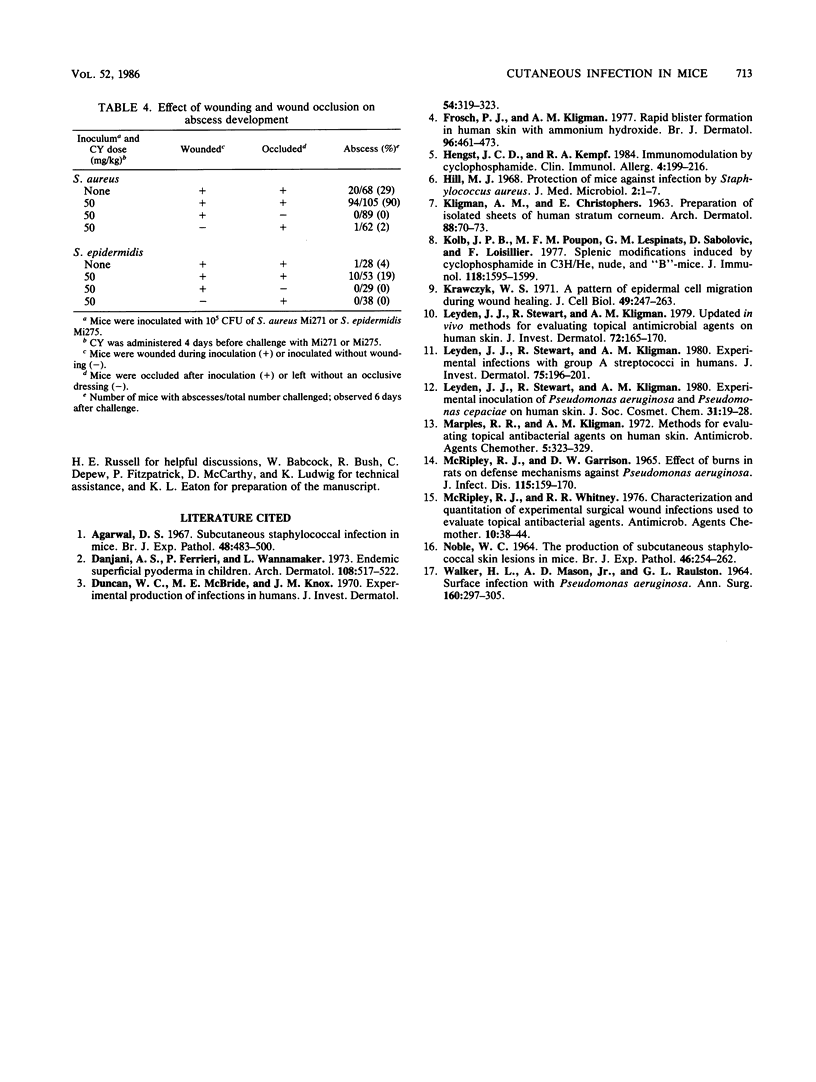
Since a model of staphylococcal skin infection adequately reflecting human disease was unavailable, a self-limiting animal infection model specific for virulent Staphylococcus species was developed. A virulent strain of S. aureus, NCTC 9789 (ATCC 27700), was used to develop an infection model in adult, male CF-1 mice treated with 0 to 150 mg of cyclophosphamide (CY) per kg 4 days before challenge. Bacteria were inoculated onto the dorsal side of shaved mice at 0 to 10(6) CFU per mouse. Simultaneously, the skin was gently scraped to remove the superficial layers without drawing blood. The wound was occluded with impermeable film secured with surgical tape. At a CY dose of 50 mg/kg and an inoculum of 10(5) CFU, 89% of the mice (96 of 108) developed large abscesses (approximately 15-mm diameter). Mice which were not immunocompromised developed fewer abscesses (20 of 68). Generally, no abscesses formed when the mice were not wounded (1 of 62), occluded (0 of 89), or inoculated (11 of 50). The abscesses developed 24 to 48 h after challenge and persisted for 2 to 3 weeks. The challenge organism was isolated from the abscesses. The rates of abscess formation of three additional S. aureus strains varied widely in normal and CY-treated mice. Three strains of S. epidermidis, one of Micrococcus varians, and one of S. saprophyticus failed to cause abscesses. Bacterial proliferation studies demonstrated that a strain of S. aureus and a strain of S. epidermidis proliferated to the same levels 48 h after challenge. Immunosuppression and wounding had little effect on the levels of proliferation of S. aureus (P greater than 0.2). Without occlusion, however, S. aureus proliferated to significantly lower levels (P less than 0.005). This model may be be useful for screening topical anti-infective agents or studying the mechanisms of bacterial pathogenesis and host response.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal D. S. Subcutaneous staphylococcal infection in mice. 3. Effect of active and passive immunization and anti-inflammatory drugs. Br J Exp Pathol. 1967 Oct;48(5):483–500. [PMC free article] [PubMed] [Google Scholar]
- Dajani A. S., Ferrieri P., Wannamaker L. Endemic superficial pyoderma in children. Arch Dermatol. 1973 Oct;108(4):517–522. [PubMed] [Google Scholar]
- Duncan W. C., McBride M. E., Knox J. M. Experimental production of infections in humans. J Invest Dermatol. 1970 Apr;54(4):319–323. doi: 10.1111/1523-1747.ep12258627. [DOI] [PubMed] [Google Scholar]
- Frosch P. J., Kligman A. M. Rapid blister formation in human skin with ammonium hydroxide. Br J Dermatol. 1977 May;96(5):461–473. doi: 10.1111/j.1365-2133.1977.tb07148.x. [DOI] [PubMed] [Google Scholar]
- Hill M. J. Protection of mice against infection by Staphylococcus aureus. J Med Microbiol. 1969 Feb;2(1):1–7. doi: 10.1099/00222615-2-1-1. [DOI] [PubMed] [Google Scholar]
- Kolb J. P., Poupon M. F., Lespinats G. M., Sabolovic D., Loisillier F. Splenic modifications induced by cyclophosphamide in C3H/He, nude, and "B" mice. J Immunol. 1977 May;118(5):1595–1599. [PubMed] [Google Scholar]
- Leyden J. J., Stewart R., Kligman A. M. Experimental infections with group A streptococci in humans. J Invest Dermatol. 1980 Aug;75(2):196–201. doi: 10.1111/1523-1747.ep12522655. [DOI] [PubMed] [Google Scholar]
- Leyden J. J., Stewart R., Kligman A. M. Updated in vivo methods for evaluating topical antimicrobial agents on human skin. J Invest Dermatol. 1979 Apr;72(4):165–170. doi: 10.1111/1523-1747.ep12676347. [DOI] [PubMed] [Google Scholar]
- MCRIPLEY R. J., GARRISON D. W. EFFECT OF BURNS IN RATS ON DEFENSE MECHANISMS AGAINST PSEUDOMONAS AERUGINOSA. J Infect Dis. 1965 Apr;115:159–170. doi: 10.1093/infdis/115.2.159. [DOI] [PubMed] [Google Scholar]
- Marples R. R., Kligman A. M. Methods for evaluating topical antibacterial agents on human skin. Antimicrob Agents Chemother. 1974 Mar;5(3):323–329. doi: 10.1128/aac.5.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRipley R. J., Whitney R. R. Characterization and quantitation of experimental surgical-wound infections used to evaluate topical antibacterial agents. Antimicrob Agents Chemother. 1976 Jul;10(1):38–44. doi: 10.1128/aac.10.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble W. C. The production of subcutaneous staphylococcal skin lesions in mice. Br J Exp Pathol. 1965 Jun;46(3):254–262. [PMC free article] [PubMed] [Google Scholar]
- WALKER H. L., MASON A. D., Jr, RAULSTON G. L. SURFACE INFECTION WITH PSEUDOMONAS AERUGINOSA. Ann Surg. 1964 Aug;160:297–305. doi: 10.1097/00000658-196408000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]




