Abstract
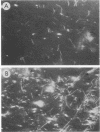
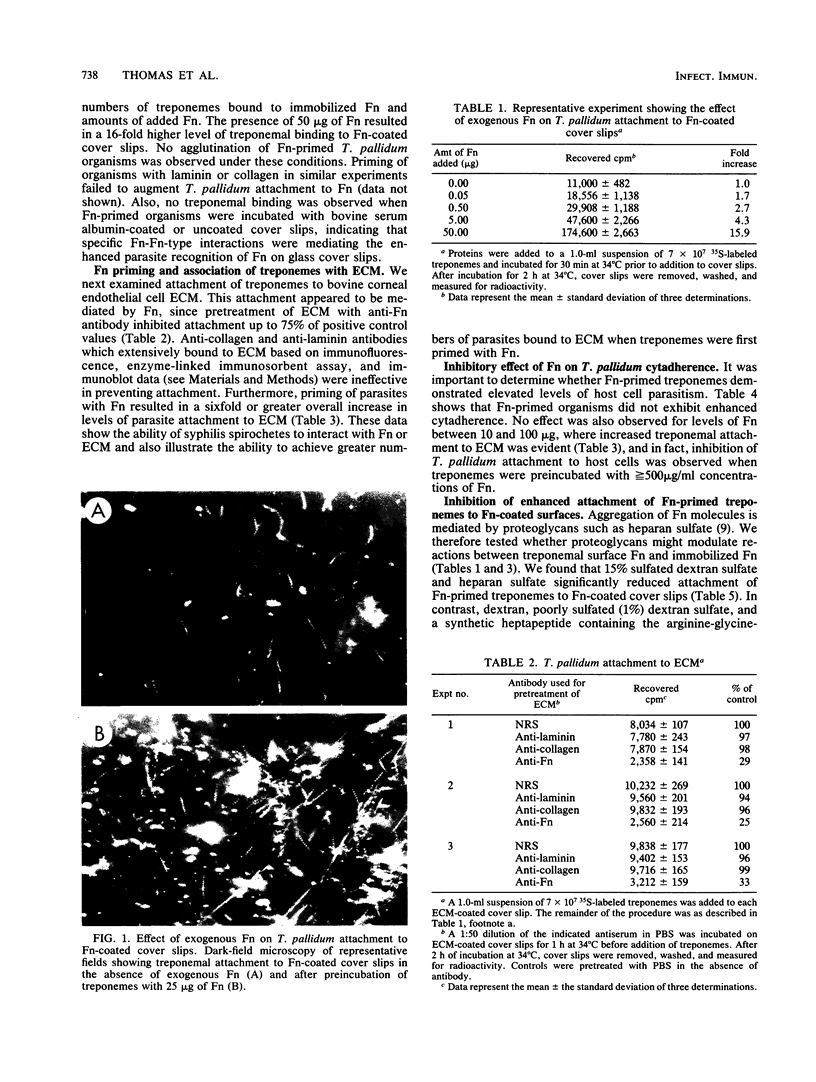
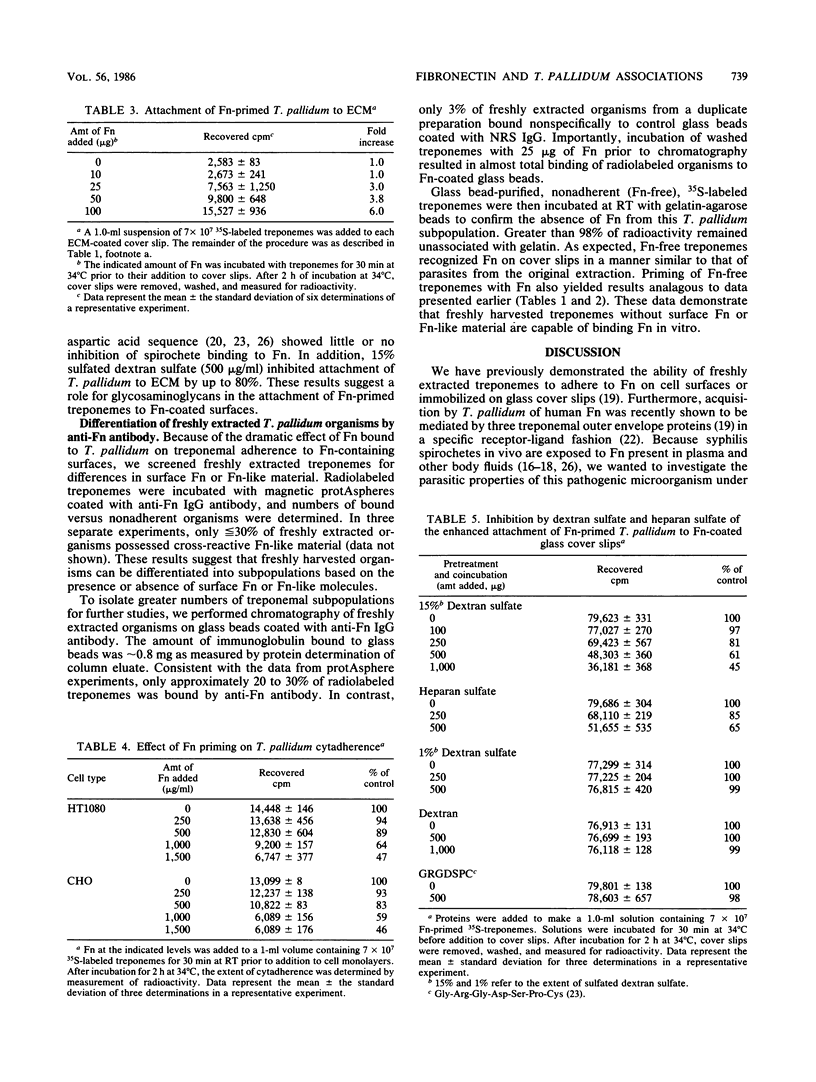
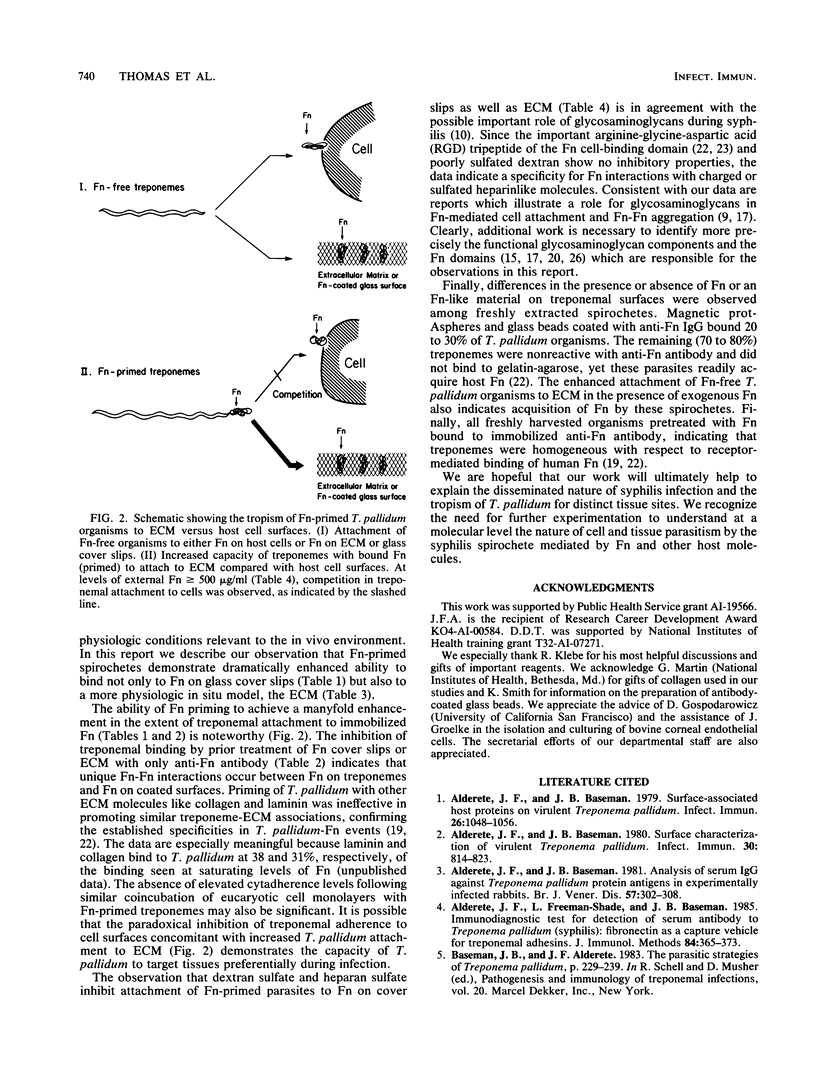
Freshly extracted Treponema pallidum organisms treated with exogenous human fibronectin (Fn) (Fn-primed treponemes) showed a 6- to 15-fold increased level of attachment to Fn-coated cover slips and to extracellular matrix (ECM) when compared with unprimed treponemes. Treponemes primed with collagen or laminin showed no similar enhanced binding to immobilized Fn or ECM. Preexposure of immobilized Fn and ECM to anti-Fn serum but not to anti-collagen or anti-laminin serum prevented treponemal adherence. Also, the presence of proteoglycanlike molecules such as dextran sulfate or heparan sulfate inhibited Fn-primed treponemal attachment to Fn or ECM. In contrast Fn-primed treponemes did not exhibit elevated levels of attachment to eucaryotic cell monolayers. To understand the increased tropism of Fn-primed T. pallidum organisms for Fn and ECM-like surfaces, we radiolabeled freshly extracted treponemes with [35S]methionine and examined them for the presence of surface immunoreactive Fn. Magnetic protAspheres and glass beads coated with monospecific anti-Fn serum bound only 20 to 30% of radiolabeled treponemes. Nonadherent treponemes failed to bind to gelatin-agarose, further confirming the absence of surface Fn or Fn-like material. Fn-free organisms, however, did attach to Fn-coated cover slips and to cell monolayers like treponemes of the original population. Incubation of Fn-free treponemes with human Fn resulted in almost total binding of organisms to anti-Fn antibody on glass beads and also produced increased attachment to Fn-coated cover slips and ECM. These results suggest that enhanced interactions between T. pallidum and the host are dependent on the presence of Fn on syphilis spirochetes and the specific location and orientation of Fn in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Analysis of serum IgG against Treponema pallidum protein antigens in experimentally infected rabbits. Br J Vener Dis. 1981 Oct;57(5):302–308. doi: 10.1136/sti.57.5.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Baseman J. B. Surface characterization of virulent Treponema pallidum. Infect Immun. 1980 Dec;30(3):814–823. doi: 10.1128/iai.30.3.814-823.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Baseman J. B. Surface-associated host proteins on virulent Treponema pallidum. Infect Immun. 1979 Dec;26(3):1048–1056. doi: 10.1128/iai.26.3.1048-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Freeman-Shade L., Baseman J. B. Immunodiagnostic test for detection of serum antibody to Treponema pallidum (syphilis): fibronectin as a capture vehicle for treponemal adhesins. J Immunol Methods. 1985 Nov 28;84(1-2):365–373. doi: 10.1016/0022-1759(85)90443-0. [DOI] [PubMed] [Google Scholar]
- Baseman J. B., Hayes E. C. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J Exp Med. 1980 Mar 1;151(3):573–586. doi: 10.1084/jem.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes N. S. Anabolic potential of virulent Treponema pallidum. Infect Immun. 1977 Dec;18(3):857–859. doi: 10.1128/iai.18.3.857-859.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes N. S. Protein synthesis by Treponema pallidum extracted from infected rabbit tissue. Infect Immun. 1974 Dec;10(6):1350–1355. doi: 10.1128/iai.10.6.1350-1355.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley K. L., Klebe R. J., Hurst R. E., Horowitz P. M. Heparin binding is necessary, but not sufficient, for fibronectin aggregation. A fluorescence polarization study. J Biol Chem. 1985 Jun 25;260(12):7250–7256. [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Miller J. N., Sykes J. A. Characterization of the attachment of Treponema pallidum (Nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect Immun. 1977 Nov;18(2):467–478. doi: 10.1128/iai.18.2.467-478.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Surface mucopolysaccharides of Treponema pallidum. Infect Immun. 1979 Apr;24(1):244–251. doi: 10.1128/iai.24.1.244-251.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Repesh L. A. Interactions of fibronectin with Treponema pallidum. Genitourin Med. 1985 Jun;61(3):147–155. doi: 10.1136/sti.61.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes N. S., Muse K. E., Collier A. M., Baseman J. B. Parasitism by virulent Treponema pallidum of host cell surfaces. Infect Immun. 1977 Jul;17(1):174–186. doi: 10.1128/iai.17.1.174-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A., Scott-Burden T., Gevers W. Glycoprotein, elastin, and collagen secretion by rat smooth muscle cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):353–357. doi: 10.1073/pnas.76.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebe R. J., Mock P. J. Effect of glycosaminoglycans on fibronectin-mediated cell attachment. J Cell Physiol. 1982 Jul;112(1):5–9. doi: 10.1002/jcp.1041120103. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Amenta P. S. The basement membrane in pathology. Lab Invest. 1983 Jun;48(6):656–677. [PubMed] [Google Scholar]
- Peterson K. M., Baseman J. B., Alderete J. F. Treponema pallidum receptor binding proteins interact with fibronectin. J Exp Med. 1983 Jun 1;157(6):1958–1970. doi: 10.1084/jem.157.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Schwarz M. A., Juliano R. L. Two distinct mechanisms for the interaction of cells with fibronectin substrata. J Cell Physiol. 1985 Jul;124(1):113–119. doi: 10.1002/jcp.1041240118. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Fibronectin mediates Treponema pallidum cytadherence through recognition of fibronectin cell-binding domain. J Exp Med. 1985 Mar 1;161(3):514–525. doi: 10.1084/jem.161.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Fibronectin tetrapeptide is target for syphilis spirochete cytadherence. J Exp Med. 1985 Nov 1;162(5):1715–1719. doi: 10.1084/jem.162.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Akiyama S. K., Hasegawa T., Hasegawa E., Humphries M. J., Kennedy D. W., Nagata K., Urushihara H., Olden K., Chen W. T. Recent advances in research on fibronectin and other cell attachment proteins. J Cell Biochem. 1985;28(2):79–97. doi: 10.1002/jcb.240280202. [DOI] [PubMed] [Google Scholar]