Abstract
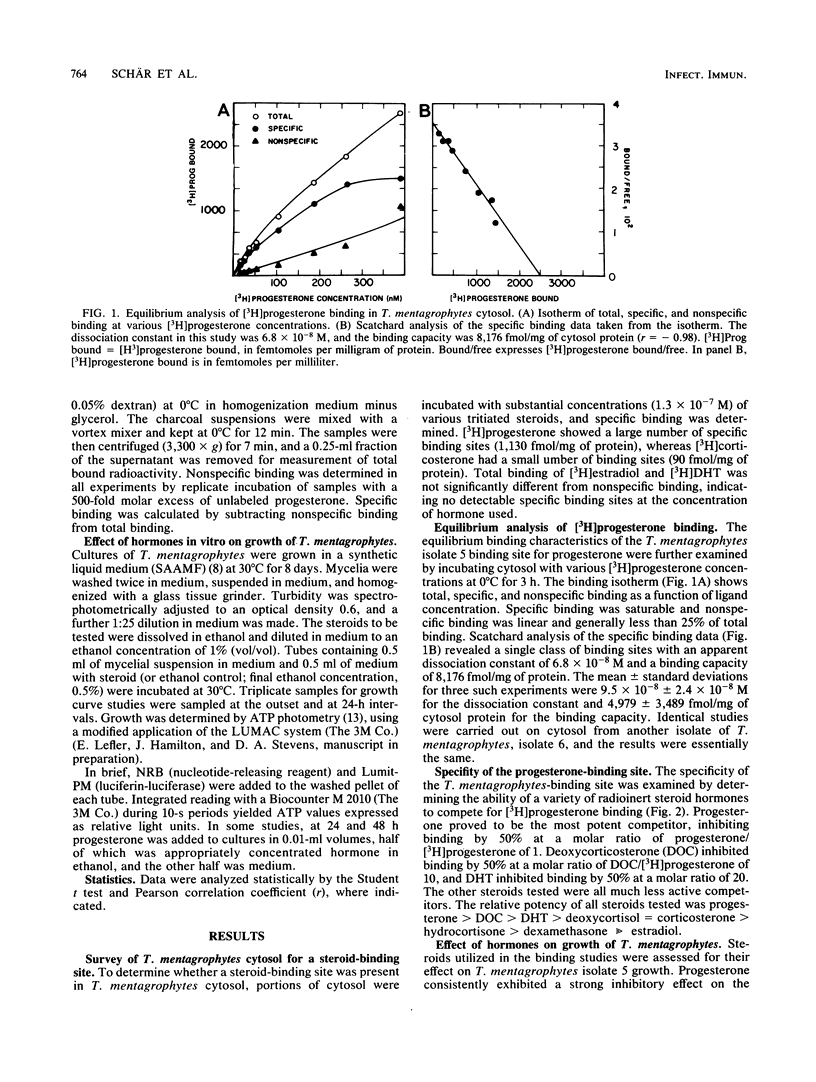
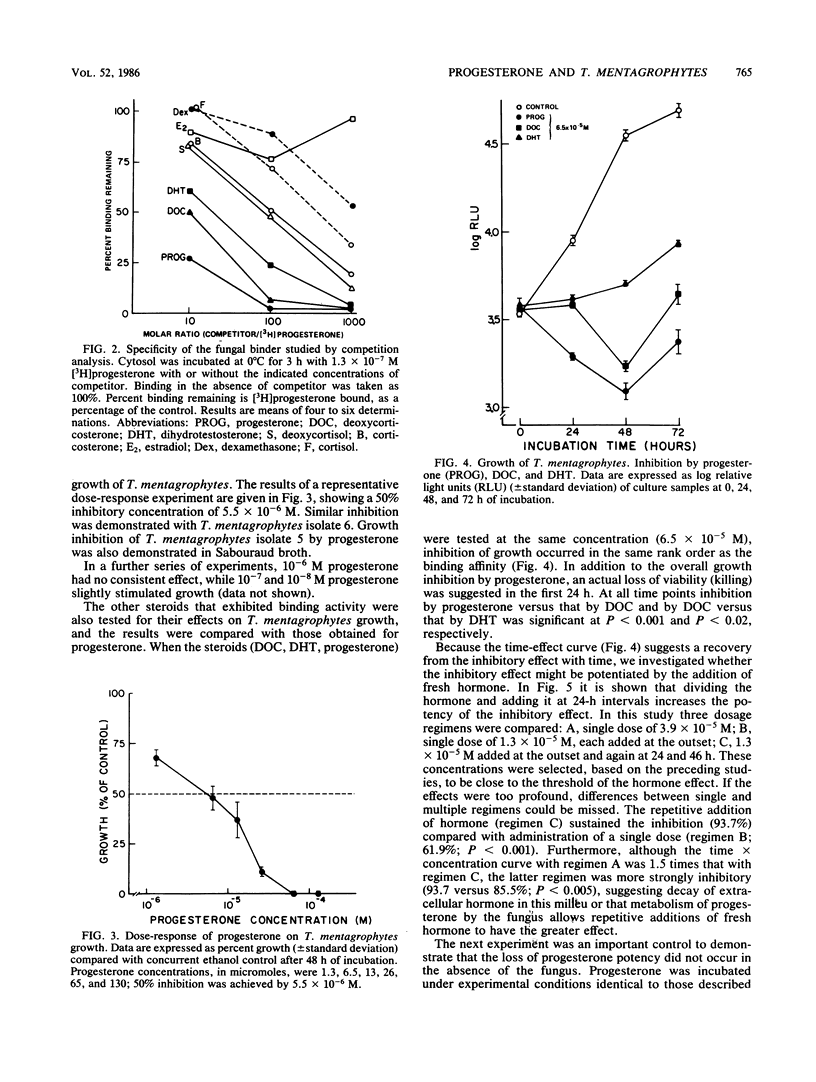
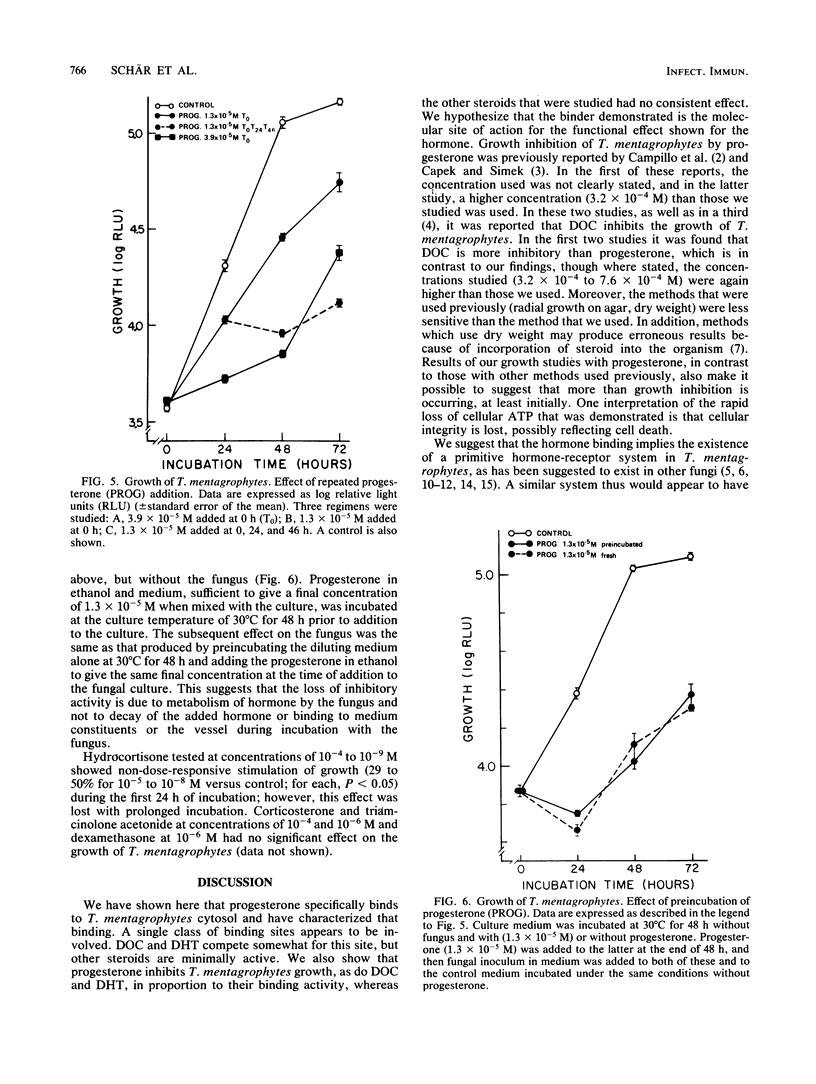
Specific binding of [3H]progesterone to cytosol of Trichophyton mentagrophytes was demonstrated. Scatchard analysis of [3H]progesterone binding showed a single class of binding sites with a dissociation constant of 9.5 X 10(-8) [corrected] +/- 2.4 X 10(-8) M (standard deviation) and a maximal binding capacity of 4,979 +/- 3,489 fmol/mg of cytosol protein. Deoxycorticosterone and dihydrotestosterone competitively inhibited binding by 50% at molar ratios of 10:1 and 20:1, respectively. Other steroid hormones that were tested had minimal activity, indicating binding specificity. Steroid hormone actions in T. mentagrophytes were examined in growth studies. Growth was assessed by determination of cellular ATP content. Progesterone inhibited growth in a dose-responsive manner, with a 50% inhibition concentration of 5.5 X 10(-6) M. Partial recovery from inhibition occurred after 24 to 48 h; inhibition could be enhanced by dividing the amount of added progesterone every 24 h. In the same rank order as was their relationship to each other and progesterone in binding studies, deoxycorticosterone and dihydrotestosterone were less effective inhibitors; other steroid hormones that were tested showed no consistent effect. We hypothesize that the binder described, acting as a hormone receptor, is the molecular site of action for the functional effect of the hormone. The functional effect may be related to the observed resistance of females to dermatophytosis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CASAS-CAMPILLO C., BALANDRANO D., GALARZA A. Steroids. 159. Antimicrobial properties of 21,21-dimethoxy progesterone and other progesterone analogues. J Bacteriol. 1961 Mar;81:366–375. doi: 10.1128/jb.81.3.366-375.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHATTAWAY F. W., TOWNSLEY J. D., BARLOW A. J. Effect of steroids and related compounds on the growth of dermatophytes. Nature. 1959 Nov 28;184(Suppl 22):1731–1732. doi: 10.1038/1841731a0. [DOI] [PubMed] [Google Scholar]
- Capek A., Simek A. Antimicrobial agents. IX. Effect of steroids on dermatophytes. Folia Microbiol (Praha) 1971;16(4):299–302. doi: 10.1007/BF02872811. [DOI] [PubMed] [Google Scholar]
- Drutz D. J., Huppert M., Sun S. H., McGuire W. L. Human sex hormones stimulate the growth and maturation of Coccidioides immitis. Infect Immun. 1981 May;32(2):897–907. doi: 10.1128/iai.32.2.897-907.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D., Do Y., Burshell A., Stathis P., Loose D. S. An estrogen-binding protein and endogenous ligand in Saccharomyces cerevisiae: possible hormone receptor system. Science. 1982 Oct 15;218(4569):297–298. doi: 10.1126/science.6289434. [DOI] [PubMed] [Google Scholar]
- Ghannoum M. A., Mudher S., Burns G. Incorporation of dexamethasone by Candida albicans. Microbios. 1985;42(168):103–109. [PubMed] [Google Scholar]
- Hoeprich P. D., Finn P. D. Obfuscation of the activity of antifungal antimicrobics by culture media. J Infect Dis. 1972 Oct;126(4):353–361. doi: 10.1093/infdis/126.4.353. [DOI] [PubMed] [Google Scholar]
- Loose D. S., Schurman D. J., Feldman D. A corticosteroid binding protein and endogenous ligand in C. albicans indicating a possible steroid-receptor system. Nature. 1981 Oct 8;293(5832):477–479. doi: 10.1038/293477a0. [DOI] [PubMed] [Google Scholar]
- Loose D. S., Stevens D. A., Schurman D. J., Feldman D. Distribution of a corticosteroid-binding protein in Candida and other fungal genera. J Gen Microbiol. 1983 Aug;129(8):2379–2385. doi: 10.1099/00221287-129-8-2379. [DOI] [PubMed] [Google Scholar]
- Loose D. S., Stover E. P., Restrepo A., Stevens D. A., Feldman D. Estradiol binds to a receptor-like cytosol binding protein and initiates a biological response in Paracoccidioides brasiliensis. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7659–7663. doi: 10.1073/pnas.80.24.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F. C. Laboratory evaluation of antifungal agents: a comparative study of five imidazole derivatives of clinical importance. J Antimicrob Chemother. 1980 Nov;6(6):749–761. doi: 10.1093/jac/6.6.749. [DOI] [PubMed] [Google Scholar]
- Powell B. L., Drutz D. J., Huppert M., Sun S. H. Relationship of progesterone- and estradiol-binding proteins in Coccidioides immitis to coccidioidal dissemination in pregnancy. Infect Immun. 1983 May;40(2):478–485. doi: 10.1128/iai.40.2.478-485.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo A., Salazar M. E., Cano L. E., Stover E. P., Feldman D., Stevens D. A. Estrogens inhibit mycelium-to-yeast transformation in the fungus Paracoccidioides brasiliensis: implications for resistance of females to paracoccidioidomycosis. Infect Immun. 1984 Nov;46(2):346–353. doi: 10.1128/iai.46.2.346-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


