Abstract
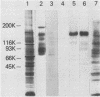
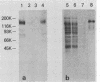
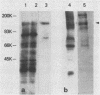
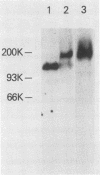
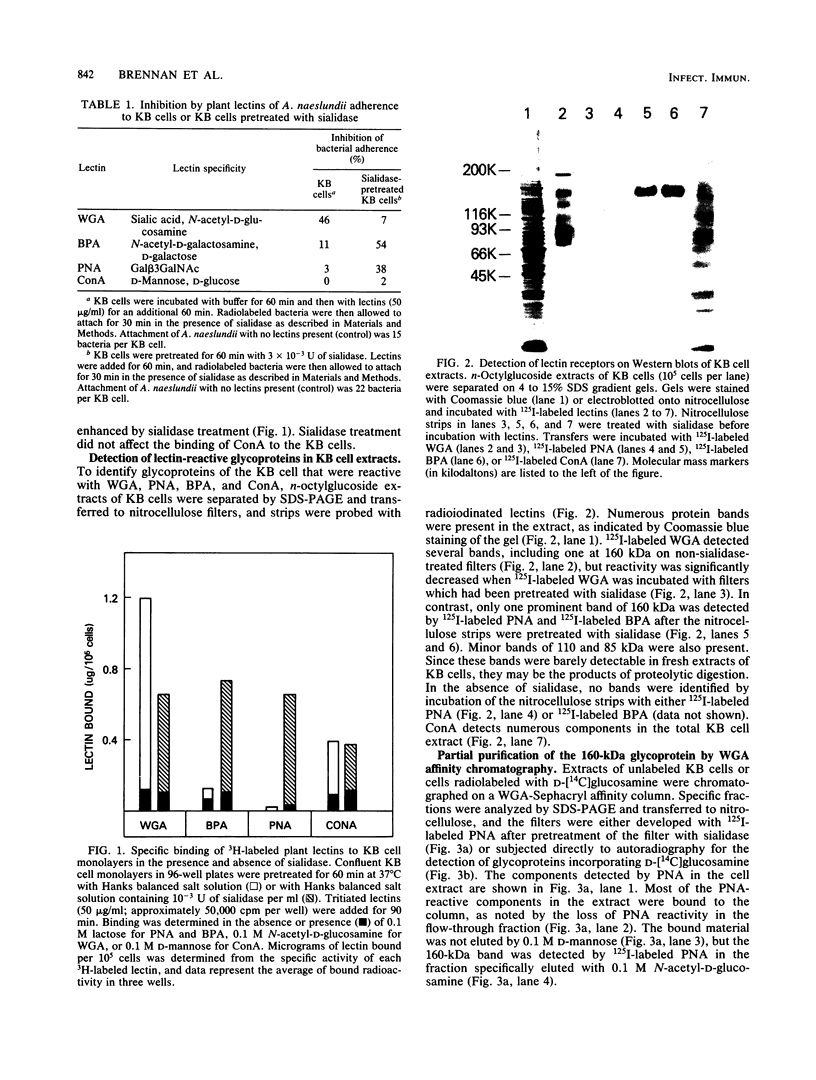
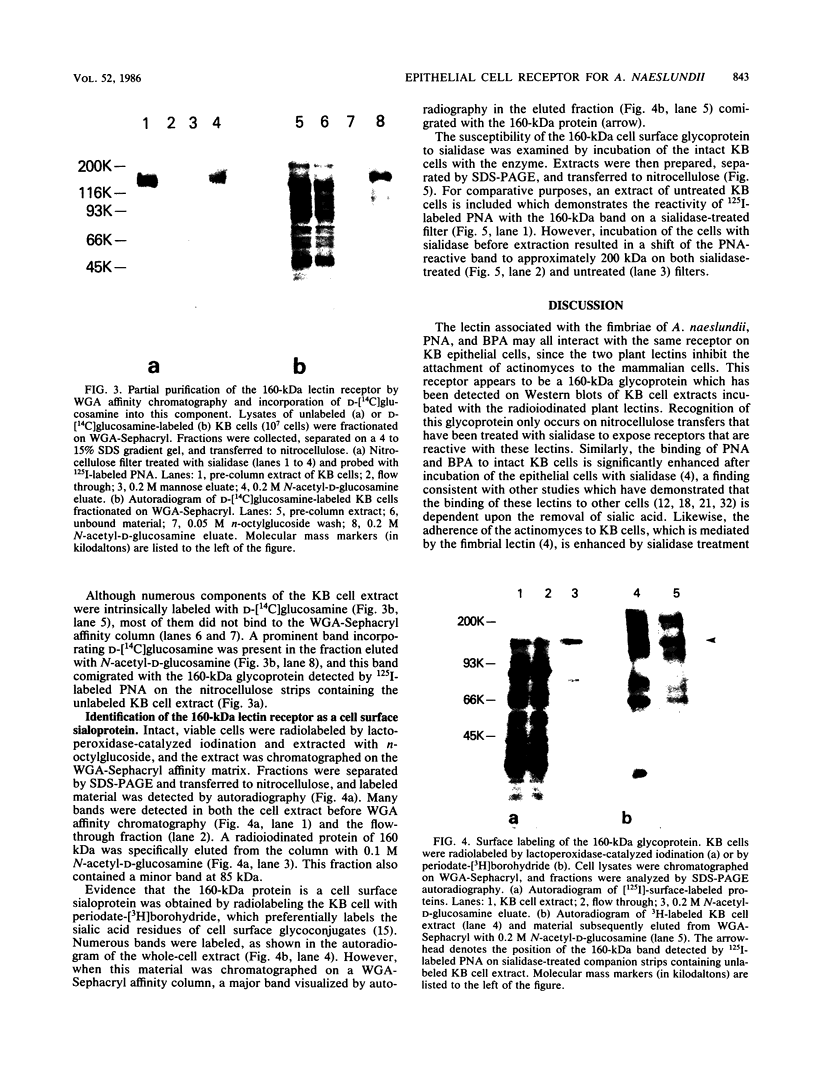
The adherence of Actinomyces naeslundii to human epithelial (KB) cells is mediated by the interaction of a fimbrial lectin on this oral bacterium with epithelial cell receptors exposed by sialidase. The D-galactose- and N-acetyl-D-galactosamine-reactive plant lectins from peanut and from Bauhinia purpurea inhibit this interaction. This report describes the partial purification and characterization of a 160-kilodalton (kDa) cell surface glycoprotein which is the principal receptor for these lectins. Radioiodinated lectins detected a band of 160 kDa on sialidase-treated Western blots of epithelial cell extracts but did not detect bands on nontreated filters. However, wheat germ agglutinin was reactive with the 160-kDa band on filters that were not treated with sialidase, suggesting that this lectin recognizes the sialic acid residues of this molecule. The 160-kDa component was partially purified from n-octylglucoside extracts of the epithelial cells by wheat germ agglutinin affinity chromatography. This molecule was metabolically labeled with D-[14C]glucosamine and labeled at the cell surface by lactoperoxidase-catalyzed iodination or periodate oxidation followed by sodium borotritide reduction. Incubation of epithelial cells with sialidase before extraction resulted in the loss of the 160-kDa band and the appearance of a band at 200 kDa which was directly reactive with 125I-labeled peanut agglutinin. These results indicate that the 160-kDa glycoprotein on the surface of the epithelial cell serves as a receptor for the agglutinins from the peanut and B. purpurea and presumably the fimbrial lectin of actinomyces.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Bhavanandan V. P., Katlic A. W. The interaction of wheat germ agglutinin with sialoglycoproteins. The role of sialic acid. J Biol Chem. 1979 May 25;254(10):4000–4008. [PubMed] [Google Scholar]
- Brennan M. J., Cisar J. O., Vatter A. E., Sandberg A. L. Lectin-dependent attachment of Actinomyces naeslundii to receptors on epithelial cells. Infect Immun. 1984 Nov;46(2):459–464. doi: 10.1128/iai.46.2.459-464.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Barsumian E. L., Curl S. H., Vatter A. E., Sandberg A. L., Siraganian R. P. Detection and localization of a lectin on Actinomyces viscosus T14V by monoclonal antibodies. J Immunol. 1981 Oct;127(4):1318–1322. [PubMed] [Google Scholar]
- Cisar J. O., David V. A., Curl S. H., Vatter A. E. Exclusive presence of lactose-sensitive fimbriae on a typical strain (WVU45) of Actinomyces naeslundii. Infect Immun. 1984 Nov;46(2):453–458. doi: 10.1128/iai.46.2.453-458.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A. H., Cisar J. O., Kolenbrander P. E., Gabriel O. Neuraminidase-dependent hamagglutination of human erythrocytes by human strains of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979 Nov;26(2):563–572. doi: 10.1128/iai.26.2.563-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S., Schneider W. J., Hobgood K. K., Tolleshaug H., Brown M. S., Goldstein J. L. Biosynthesis of N- and O-linked oligosaccharides of the low density lipoprotein receptor. J Biol Chem. 1983 Dec 25;258(24):15261–15273. [PubMed] [Google Scholar]
- Dzandu J. K., Deh M. E., Barratt D. L., Wise G. E. Detection of erythrocyte membrane proteins, sialoglycoproteins, and lipids in the same polyacrylamide gel using a double-staining technique. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1733–1737. doi: 10.1073/pnas.81.6.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Fillery E. D., Chan K. H., Grove D. A. Sialidase-enhanced lectin-like mechanism for Actinomyces viscosus and Actinomyces naeslundii hemagglutination. Infect Immun. 1980 Feb;27(2):335–343. doi: 10.1128/iai.27.2.335-343.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar G. H., Holz G., Uhlenbruck G. Glycoproteins containing peanut-agglutinin receptors from human-peripheral-blood T-lymphocyte plasma membranes. Eur J Biochem. 1981 Dec;121(1):237–241. doi: 10.1111/j.1432-1033.1981.tb06454.x. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Marchesi V. T. Glycophorins: isolation, orientation, and localization of specific domains. Methods Enzymol. 1983;96:268–280. doi: 10.1016/s0076-6879(83)96025-1. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J Biol Chem. 1977 Aug 25;252(16):5888–5894. [PubMed] [Google Scholar]
- Heeb M. J., Marini A. M., Gabriel O. Factors affecting binding of galacto ligands to Actinomyces viscosus lectin. Infect Immun. 1985 Jan;47(1):61–67. doi: 10.1128/iai.47.1.61-67.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe C. L., Lis H., Sharon N. Identification of peanut agglutinin receptors on human erythrocyte ghosts by affinity crosslinking using a new cleavable heterobifunctional reagent. Biochem Biophys Res Commun. 1979 Nov 28;91(2):402–409. doi: 10.1016/0006-291x(79)91536-5. [DOI] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- Kaifu R., Osawa T. Syntheses of O-beta-D-galactopyranosyl-(1 leads to 3)-0-(2-acetamido-2-deoxy-alpha(and -beta)-D-galactopyranosyl)-N-tosyl-L-serine and their interaction with D-galactose-binding lectins. Carbohydr Res. 1979 Mar;69:79–88. doi: 10.1016/s0008-6215(00)85753-5. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T., Osawa T. Elucidation of lectin receptors by quantitative inhibition of lectin binding to human erythrocytes and lymphocytes. Biochemistry. 1976 Oct 19;15(21):4581–4586. doi: 10.1021/bi00666a006. [DOI] [PubMed] [Google Scholar]
- Kerjaschki D., Sharkey D. J., Farquhar M. G. Identification and characterization of podocalyxin--the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol. 1984 Apr;98(4):1591–1596. doi: 10.1083/jcb.98.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S., Ginsburg V. The metabolism of glucosamine by tissue culture cells. Exp Cell Res. 1966 Mar;41(3):592–600. doi: 10.1016/s0014-4827(66)80109-x. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Canellakis E. S. Isolation and properties of gamma protein, the major transmembrane sialoglycoprotein of the HeLa cell. J Supramol Struct. 1979;12(4):435–455. doi: 10.1002/jss.400120404. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebien T. W., Boué D. R., Bradley J. G., Kersey J. H. Antibody affinity may influence antigenic modulation of the common acute lymphoblastic leukemia antigen in vitro. J Immunol. 1982 Nov;129(5):2287–2292. [PubMed] [Google Scholar]
- Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem. 1975 Nov 10;250(21):8518–8523. [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Barlow J. J., Matta K. L. Structural preferences of beta-galactoside-reactive lectins on Actinomyces viscosus T14V and Actinomyces naeslundii WVU45. Infect Immun. 1983 Aug;41(2):848–850. doi: 10.1128/iai.41.2.848-850.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. E., Kabat E. A., Lotan R., Sharon N. Immunochemical studies on the specificity of the peanut (Arachis hypogaea) agglutinin. Carbohydr Res. 1976 Oct;51(1):107–118. doi: 10.1016/s0008-6215(00)84040-9. [DOI] [PubMed] [Google Scholar]
- Peters B. P., Ebisu S., Goldstein I. J., Flashner M. Interaction of wheat germ agglutinin with sialic acid. Biochemistry. 1979 Nov 27;18(24):5505–5511. doi: 10.1021/bi00591a038. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Sharon N. Lectin receptors as lymphocyte surface markers. Adv Immunol. 1983;34:213–298. doi: 10.1016/s0065-2776(08)60380-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Kabat E. A., Gruezo F. G., Allen H. J. Immunochemical studies on the combining site of the D-galactopyranose and 2-acetamido-2-deoxy-D-galactopyranose specific lectin isolated from Bauhinia purpurea alba seeds. Arch Biochem Biophys. 1980 Oct 15;204(2):622–639. doi: 10.1016/0003-9861(80)90074-0. [DOI] [PubMed] [Google Scholar]