Abstract
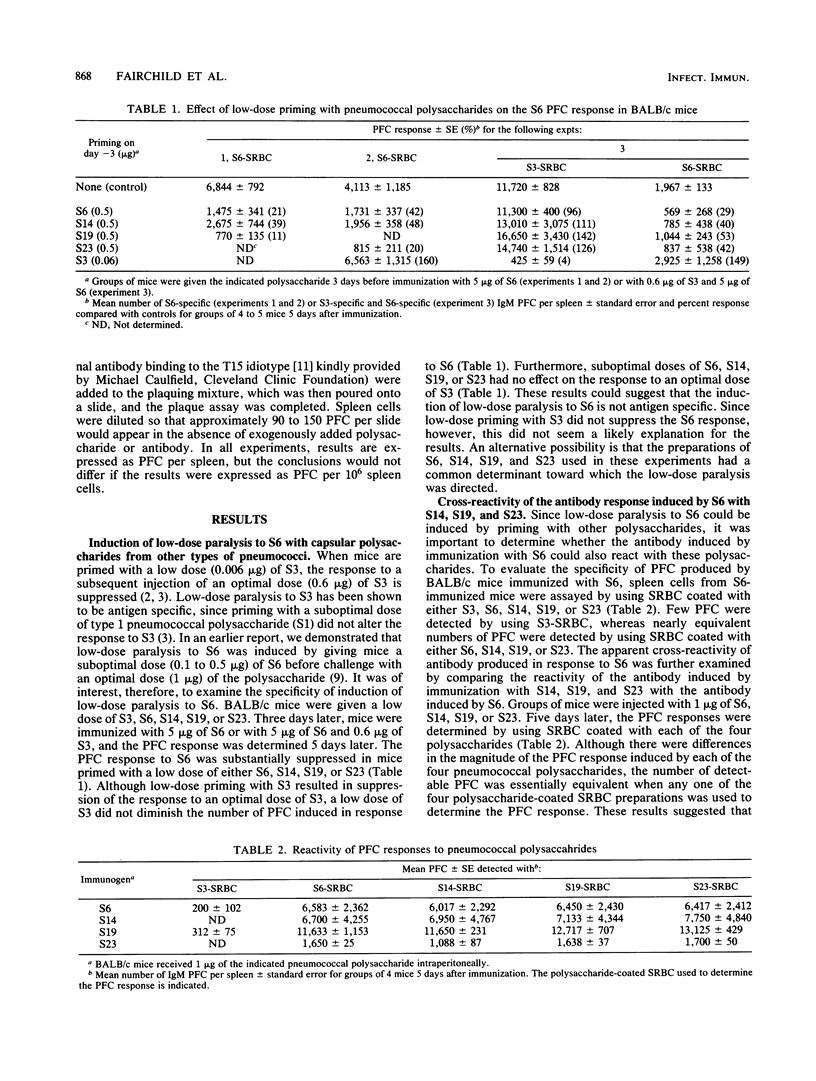
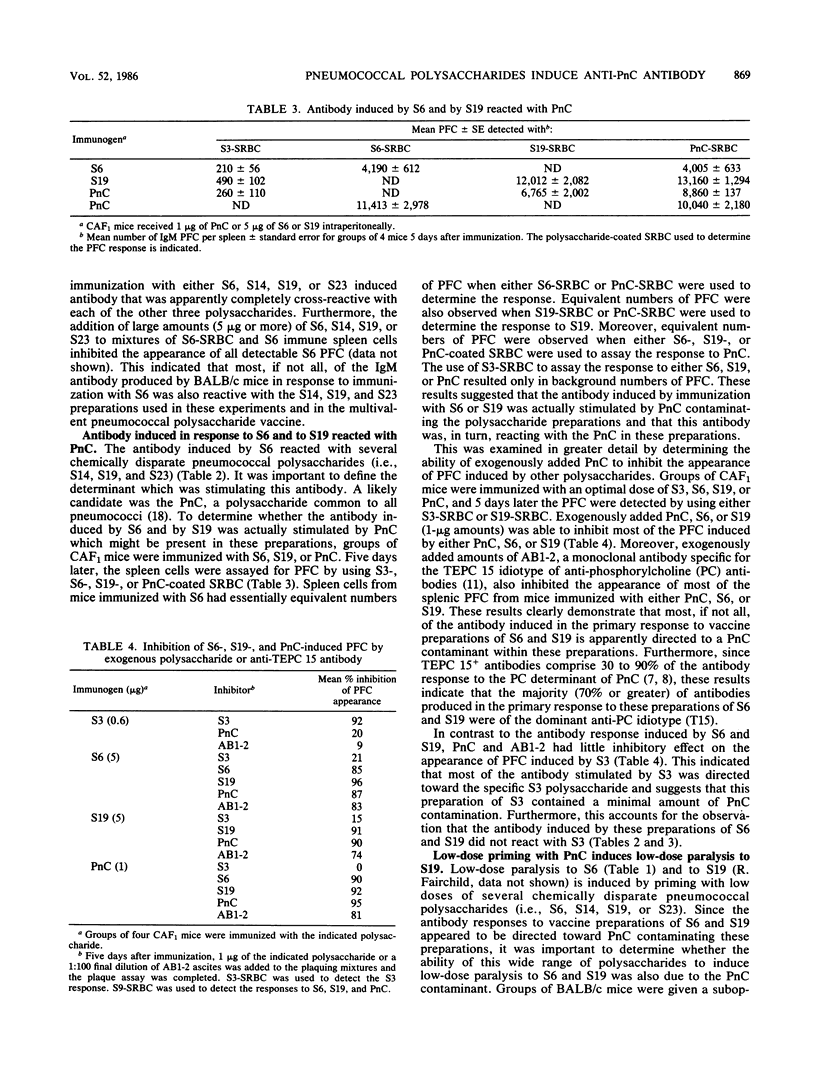
The antibody induced in mice immunized with a vaccine preparation of type 6 (Danish type 6A) pneumococcal capsular polysaccharide (S6) reacted with several chemically disparate pneumococcal capsular polysaccharides. Equivalent numbers of plaque-forming cells were observed when sheep erythrocytes coated with either S6, type 19 pneumococcal capsular polysaccharide (S19), or the pneumococcal cell wall carbohydrate (PnC) were used to detect the response to S6 or to S19. The addition of exogenous PnC to the plaquing mixtures of spleen cells from S6-, S19-, or PnC-immunized mice inhibited the appearance of most (greater than or equal to 85%) of the plaque-forming cells. Furthermore, the addition of monoclonal antibody specific for the dominant (TEPC 15) idiotype of anti-phosphorylcholine (a component of PnC) antibodies also inhibited the appearance of most of the plaque-forming cells. A suppressed S19 response was induced by priming mice with a low dose of S19 or PnC 3 days before immunization with an optimal dose of S19 (low-dose paralysis). These results demonstrated that most, if not all, of the antibody stimulated by these preparations of S6 and S19 was actually induced by and was specific for PnC.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B. Direct evidence for the involvement of T suppressor cells in the expression of low-dose paralysis to type III pneumococcal polysaccharide. J Immunol. 1982 Mar;128(3):1059–1062. [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971 Apr;20(4):469–480. [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Regulation of the antibody response to type 3 pneumococcal polysaccharide. IV. Role of suppressor T cells in the development of low-dose paralysis. J Immunol. 1974 Jun;112(6):2020–2027. [PubMed] [Google Scholar]
- Braley-Mullen H., Sharp G. C. Conversion of type III pneumococcal polysaccharide low responders to high responders by immunization with a thymus-dependent form of antigen. Cell Immunol. 1974 Apr;12(1):49–60. doi: 10.1016/0008-8749(74)90055-0. [DOI] [PubMed] [Google Scholar]
- Braley H. C., Freeman M. J. Strain differences in the antibody plaque-forming cell responses of inbred mice to pneumococcal polysaccharide. Cell Immunol. 1971 Feb;2(1):73–81. doi: 10.1016/0008-8749(71)90026-8. [DOI] [PubMed] [Google Scholar]
- Caulfield M. J. Idiotype-restricted antibody response to specific immune complexes. Cell Immunol. 1985 Feb;90(2):451–457. doi: 10.1016/0008-8749(85)90209-6. [DOI] [PubMed] [Google Scholar]
- Claflin J. L., Cubberley M. Clonal nature of the immune response to phosphocholine. VII. Evidence throughout inbred mice for molecular similarities among antibodies bearing the T15 idiotype. J Immunol. 1980 Aug;125(2):551–558. [PubMed] [Google Scholar]
- Claflin J. L. Uniformity in the clonal repertoire for the immune response to phosphorylcholine in mice. Eur J Immunol. 1976 Oct;6(10):669–674. doi: 10.1002/eji.1830061002. [DOI] [PubMed] [Google Scholar]
- Fairchild R. L., Braley-Mullen H. Characterization of the murine immune response to type 6 pneumococcal polysaccharide. Infect Immun. 1983 Feb;39(2):615–622. doi: 10.1128/iai.39.2.615-622.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Barletta R., Quan Z. S., Quintáns J. Monoclonal vs. heterogeneous anti-H-8 antibodies in the analysis of the anti-phosphorylcholine response in BALB/c mice. Eur J Immunol. 1981 Nov;11(11):877–883. doi: 10.1002/eji.1830111106. [DOI] [PubMed] [Google Scholar]
- Lake J. P., Reed N. D. Characterization of antigen-specific immunologic paralysis induced by a single low dose of polyvinylpyrrolidone. J Reticuloendothel Soc. 1976 Oct;20(4):307–316. [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen F. K., Henrichsen J. Detection of antibodies to pneumococcal capsular polysaccharides by enzyme-linked immunosorbent assay. J Clin Microbiol. 1982 Mar;15(3):372–378. doi: 10.1128/jcm.15.3.372-378.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen F. K., Henrichsen J., Sørensen U. S., Nielsen J. L. Anti-C-carbohydrate antibodies after pneumococcal vaccination. Acta Pathol Microbiol Immunol Scand C. 1982 Dec;90(6):353–355. doi: 10.1111/j.1699-0463.1982.tb01462.x. [DOI] [PubMed] [Google Scholar]
- Sørensen U. B., Agger R., Bennedsen J., Henrichsen J. Phosphorylcholine determinants in six pneumococcal capsular polysaccharides detected by monoclonal antibody. Infect Immun. 1984 Mar;43(3):876–878. doi: 10.1128/iai.43.3.876-878.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen U. B., Henrichsen J. C-polysaccharide in a pneumococcal vaccine. Acta Pathol Microbiol Immunol Scand C. 1984 Dec;92(6):351–356. doi: 10.1111/j.1699-0463.1984.tb00099.x. [DOI] [PubMed] [Google Scholar]


