Abstract
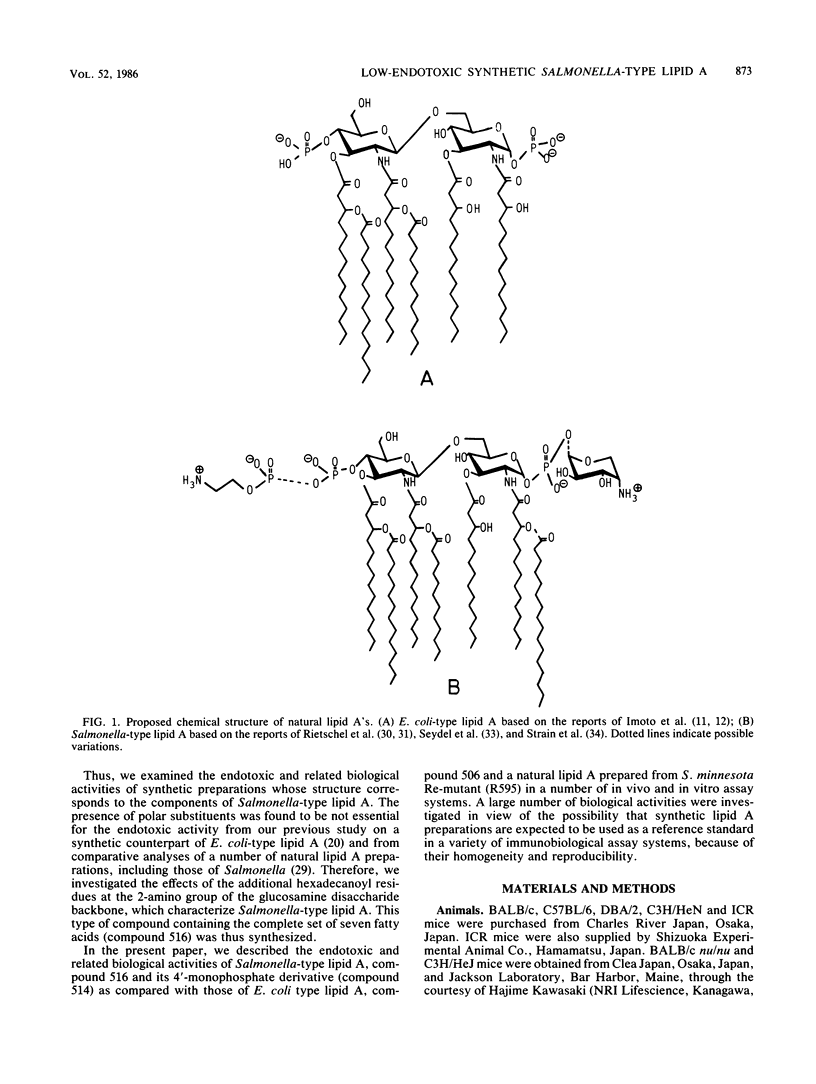
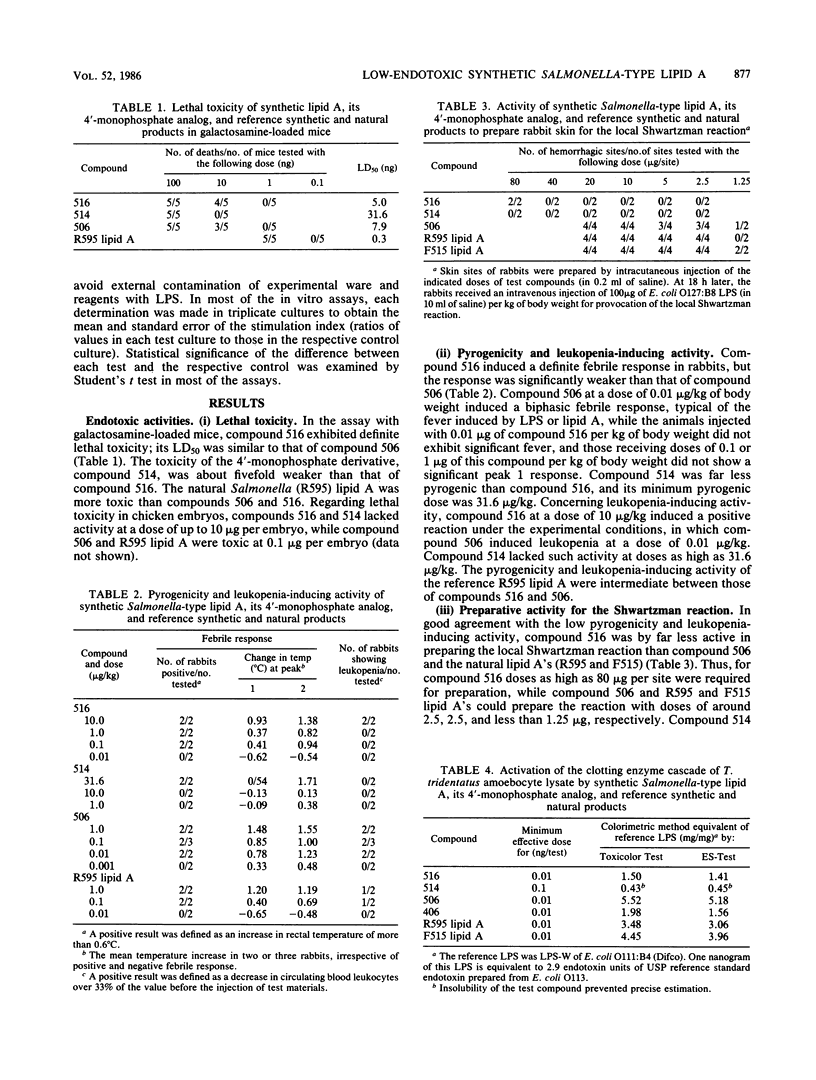
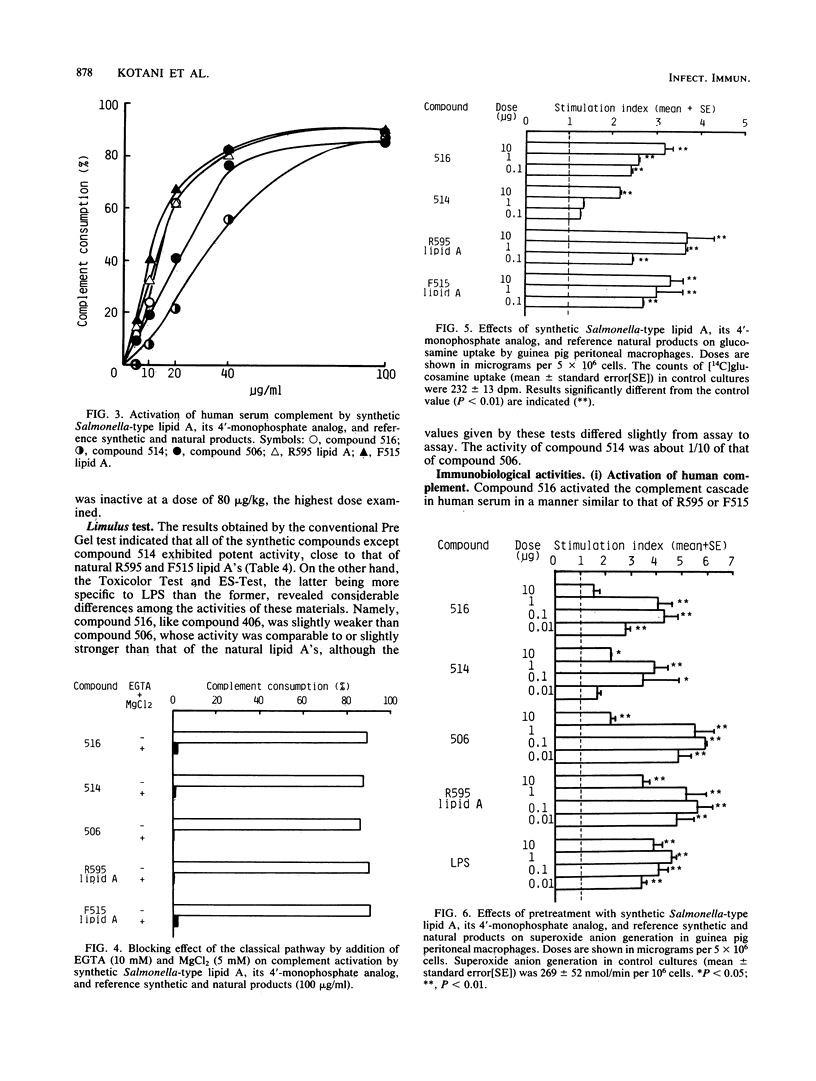
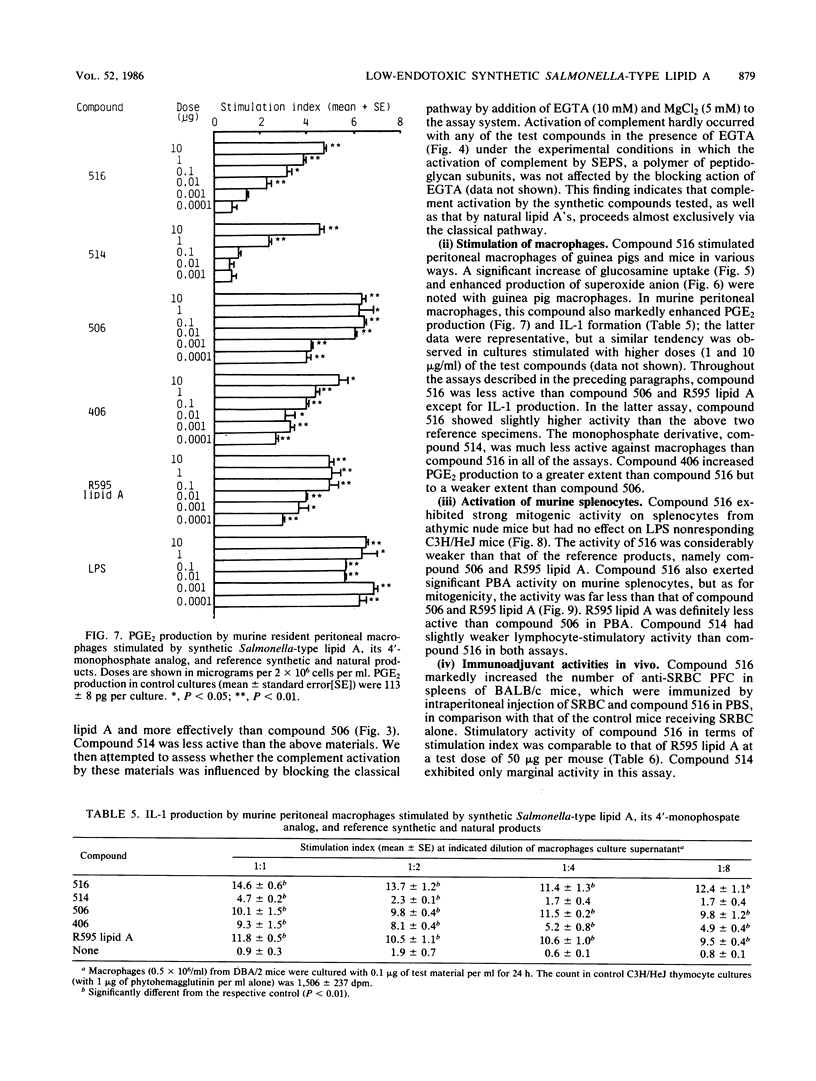
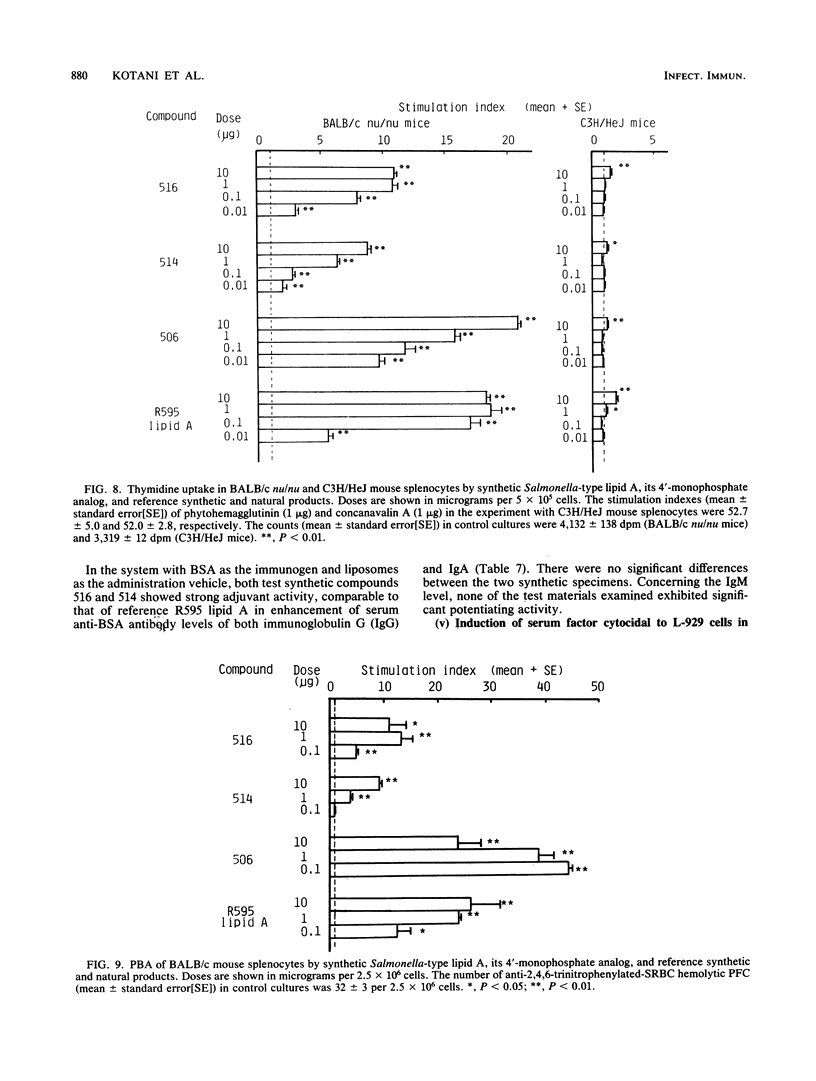
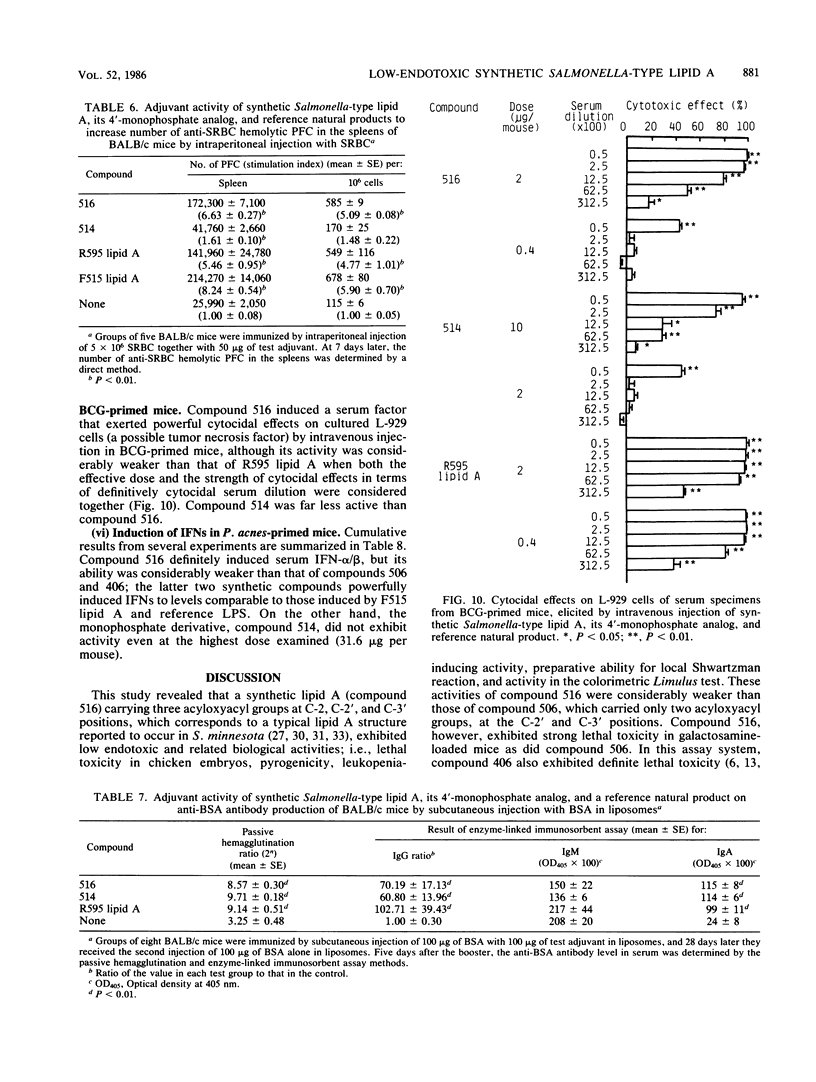
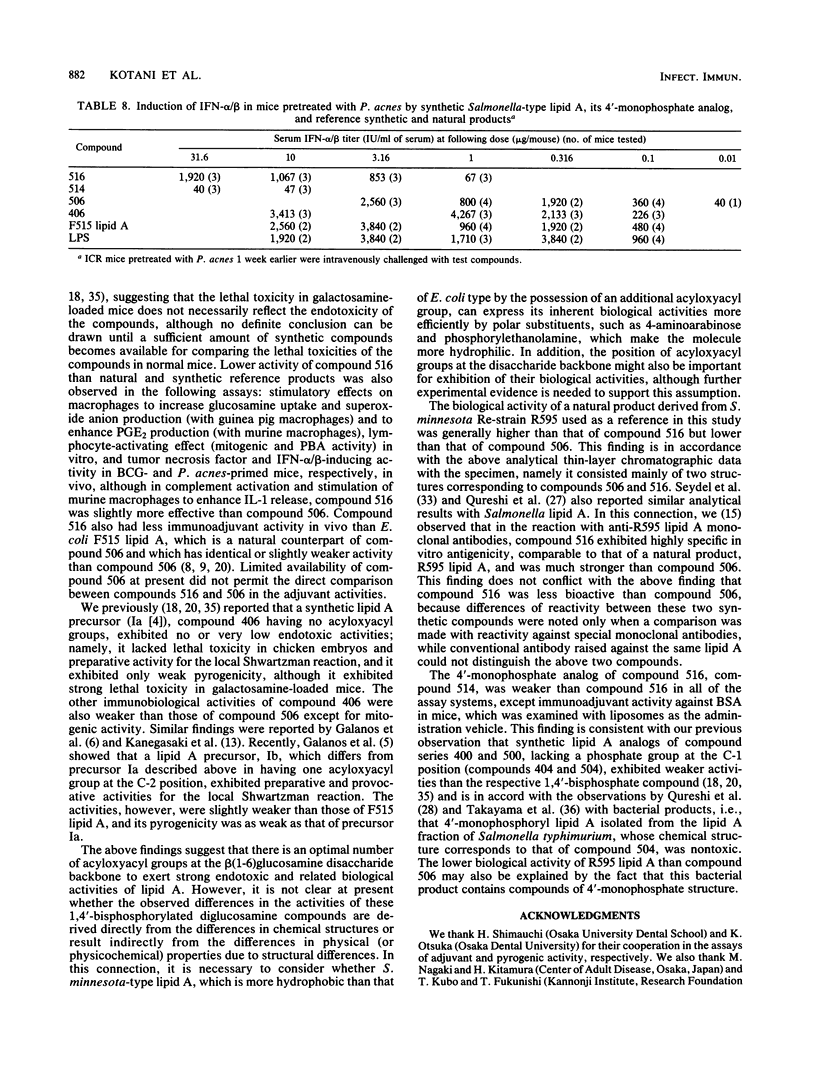
A synthetic lipid A (Salmonella type, compound 516), beta (1-6)-linked D-glucosamine disaccharide 1,4'-bisphosphate, with three acyloxyacyl groups and one hydroxyacyl group, i.e., (R)-3-hexadecanoyloxytetradecanoyl, (R)-3-hydroxytetradecanoyl, (R)-3-dodecanoyloxytetradecanoyl, and (R)-3-tetradecanoyloxytetradecanoyl groups at the 2-amino, 3-hydroxyl, 2'-amino, and 3'-hydroxyl groups, respectively, was less biologically active than the synthetic Escherichia coli-type lipid A (compound 506), which has only two acyloxyacyl groups at the 2' and 3' positions and is substituted with a (R)-3-hydroxytetradecanoyl group at the 2-amino group. Compound 516 exhibited considerably weaker pyrogenic and leukopenic activity than compound 506, and it scarcely prepared rabbit skin for the Shwartzman reaction and lacked lethal toxicity on chicken embryos, although its lethal toxicity in galactosamine-loaded mice was as strong as that of compound 506. Compound 516 was also less active than compound 506 or natural E. coli lipid A (from Restrain F515) in other biological test systems, such as the Limulus test, stimulation of macrophages and lymphocytes, and interferon-inducing activity but not for interleukin-1 induction or complement activation. This observation suggests that there is an optimal number of acyloxyacyl groups on the glucosamine backbone for producing the biological activities of lipid A, especially the endotoxic activities. The 4'-monophosphate analog (compound 514) of compound 516 in general had significantly weaker activity than compound 516 in the above assays, most probably because of its greater hydrophobicity and consequently lower solubility in assay systems. Bacterial R595 lipid A derived from S. minnesota Re-mutant, which is a mixture of compounds 516 and 506, their 4'-monophosphate analogs and other compounds, exerted intermediate degrees of activity between compounds 506 and 516 in the various test systems employed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa K., Kasai N. Endotoxic glycolipid as a potent depressor of the hepatic drug-metabolizing enzyme systems in mice. Microbiol Immunol. 1979;23(2):87–94. doi: 10.1111/j.1348-0421.1979.tb00444.x. [DOI] [PubMed] [Google Scholar]
- FINKELSTEIN R. A. OBSERVATIONS ON MODE OF ACTION OF ENDOTOXIN IN CHICK EMBRYOS. Proc Soc Exp Biol Med. 1964 Mar;115:702–707. doi: 10.3181/00379727-115-29012. [DOI] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Hansen-Hagge T., Lehmann V., Lüderitz O. Comparison of the capacity of two lipid A precursor molecules to express the local Shwartzman phenomenon. Infect Immun. 1985 May;48(2):355–358. doi: 10.1128/iai.48.2.355-358.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Rietschel E. T., Westphal O., Brade H., Brade L., Freudenberg M., Schade U., Imoto M., Yoshimura H. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur J Biochem. 1985 Apr 1;148(1):1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- Homma J. Y., Matsuura M., Kanegasaki S., Kawakubo Y., Kojima Y., Shibukawa N., Kumazawa Y., Yamamoto A., Tanamoto K., Yasuda T. Structural requirements of lipid A responsible for the functions: a study with chemically synthesized lipid A and its analogues. J Biochem. 1985 Aug;98(2):395–406. doi: 10.1093/oxfordjournals.jbchem.a135294. [DOI] [PubMed] [Google Scholar]
- Kanegasaki S., Kojima Y., Matsuura M., Homma J. Y., Yamamoto A., Kumazawa Y., Tanamoto K., Yasuda T., Tsumita T., Imoto M. Biological activities of analogues of lipid A based chemically on the revised structural model. Comparison of mediator-inducing, immunomodulating and endotoxic activities. Eur J Biochem. 1984 Sep 3;143(2):237–242. doi: 10.1111/j.1432-1033.1984.tb08364.x. [DOI] [PubMed] [Google Scholar]
- Kasai N., Arata S., Mashimo J., Okuda K., Aihara Y., Kotani S., Takada H., Shiba T., Kusumoto S., Imoto M. Synthetic Salmonella-type lipid A antigen with high serological specificity. Infect Immun. 1986 Jan;51(1):43–48. doi: 10.1128/iai.51.1.43-48.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Harada K., Mori Y., Kawasaki A., Tanaka A., Nagao S., Tanaka S. Immunobiologically active lipid A analogs synthesized according to a revised structural model of natural lipid A. Infect Immun. 1984 Jul;45(1):293–296. doi: 10.1128/iai.45.1.293-296.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Takahashi I., Ikeda T., Otsuka K., Shimauchi H., Kasai N., Mashimo J. Synthetic lipid A with endotoxic and related biological activities comparable to those of a natural lipid A from an Escherichia coli re-mutant. Infect Immun. 1985 Jul;49(1):225–237. doi: 10.1128/iai.49.1.225-237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N. Production of an anti-tumour cytotoxin by human monocytes. Immunology. 1981 Sep;44(1):135–142. [PMC free article] [PubMed] [Google Scholar]
- Nishijima M., Amano F., Akamatsu Y., Akagawa K., Tokunaga T., Raetz C. R. Macrophage activation by monosaccharide precursors of Escherichia coli lipid A. Proc Natl Acad Sci U S A. 1985 Jan;82(2):282–286. doi: 10.1073/pnas.82.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obayashi T. Addition of perchloric acid to blood samples for colorimetric limulus test using chromogenic substrate: comparison with conventional procedures and clinical applications. J Lab Clin Med. 1984 Sep;104(3):321–330. [PubMed] [Google Scholar]
- Obayashi T., Tamura H., Tanaka S., Ohki M., Takahashi S., Arai M., Masuda M., Kawai T. A new chromogenic endotoxin-specific assay using recombined limulus coagulation enzymes and its clinical applications. Clin Chim Acta. 1985 Jun 30;149(1):55–65. doi: 10.1016/0009-8981(85)90273-6. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Kotani S., Shimauchi H. Enhancement of serum antibody production in mice by oral administration of lipophilic derivatives of muramylpeptides and bacterial lipopolysaccharides with bovine serum albumin. Methods Find Exp Clin Pharmacol. 1986 Jan;8(1):19–26. [PubMed] [Google Scholar]
- Okamura H., Kawaguchi K., Shoji K., Kawade Y. High-level induction of gamma interferon with various mitogens in mice pretreated with Propionibacterium acnes. Infect Immun. 1982 Nov;38(2):440–443. doi: 10.1128/iai.38.2.440-443.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J Biol Chem. 1982 Oct 10;257(19):11808–11815. [PubMed] [Google Scholar]
- Rietschel E. T., Wollenweber H. W., Russa R., Brade H., Zähringer U. Concepts of the chemical structure of lipid A. Rev Infect Dis. 1984 Jul-Aug;6(4):432–438. doi: 10.1093/clinids/6.4.432. [DOI] [PubMed] [Google Scholar]
- Schuster B. G., Neidig M., Alving B. M., Alving C. R. Production of antibodies against phosphocholine, phosphatidylcholine, sphingomyelin, and lipid A by injection of liposomes containing lipid A. J Immunol. 1979 Mar;122(3):900–905. [PubMed] [Google Scholar]
- Seydel U., Lindner B., Wollenweber H. W., Rietschel E. T. Structural studies on the lipid A component of enterobacterial lipopolysaccharides by laser desorption mass spectrometry. Location of acyl groups at the lipid A backbone. Eur J Biochem. 1984 Dec 17;145(3):505–509. doi: 10.1111/j.1432-1033.1984.tb08585.x. [DOI] [PubMed] [Google Scholar]
- Strain S. M., Armitage I. M., Anderson L., Takayama K., Qureshi N., Raetz C. R. Location of polar substituents and fatty acyl chains on lipid A precursors from a 3-deoxy-D-manno-octulosonic acid-deficient mutant of Salmonella typhimurium. Studies by 1H, 13C, and 31P nuclear magnetic resonance. J Biol Chem. 1985 Dec 25;260(30):16089–16098. [PubMed] [Google Scholar]
- Takada H., Kotani S., Tsujimoto M., Ogawa T., Takahashi I., Harada K., Katsukawa C., Tanaka S., Shiba T., Kusumoto S. Immunopharmacological activities of a synthetic counterpart of a biosynthetic lipid A precursor molecule and of its analogs. Infect Immun. 1985 Apr;48(1):219–227. doi: 10.1128/iai.48.1.219-227.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Raetz C. R., Ribi E., Peterson J., Cantrell J. L., Pearson F. C., Wiggins J., Johnson A. G. Influence of fine structure of lipid A on Limulus amebocyte lysate clotting and toxic activities. Infect Immun. 1984 Aug;45(2):350–355. doi: 10.1128/iai.45.2.350-355.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacheron F., Guenounou M., Nauciel C. Induction of interleukin 1 secretion by adjuvant-active peptidoglycans. Infect Immun. 1983 Dec;42(3):1049–1054. doi: 10.1128/iai.42.3.1049-1054.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal O., Jann K., Himmelspach K. Chemistry and immunochemistry of bacterial lipopolysaccharides as cell wall antigens and endotoxins. Prog Allergy. 1983;33:9–39. [PubMed] [Google Scholar]


