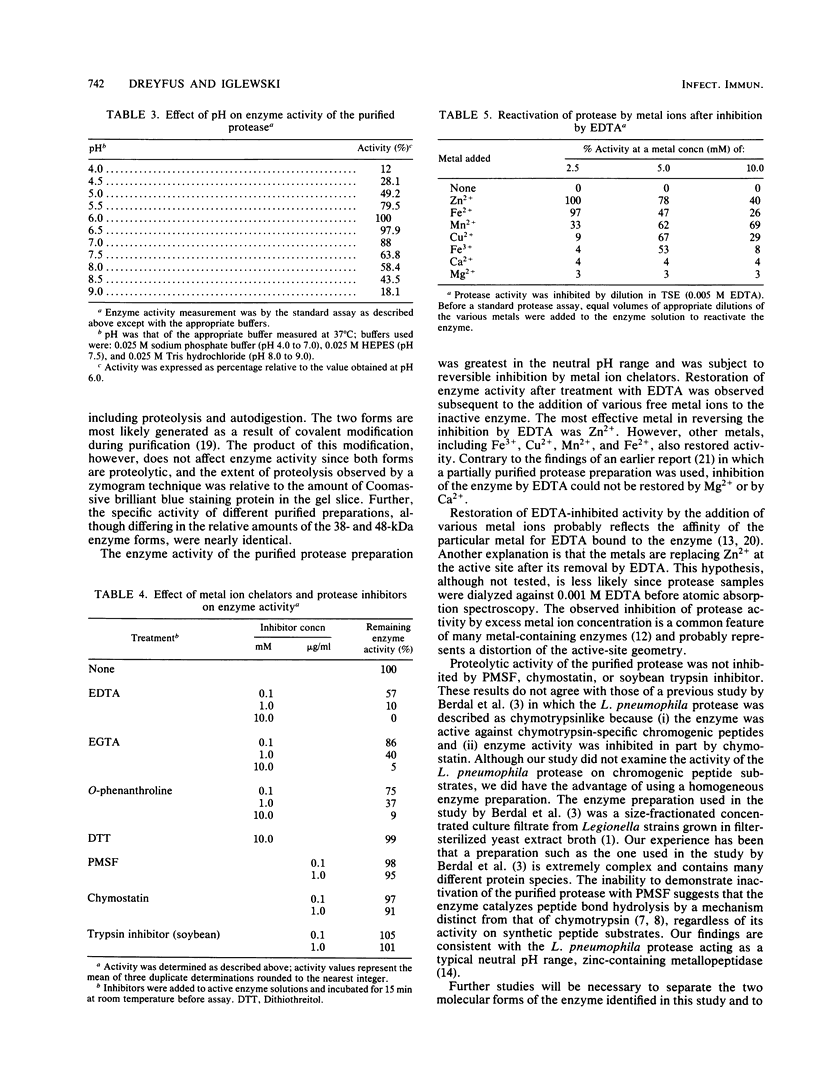
Abstract
An extracellular proteolytic enzyme of Legionella pneumophila was purified by sequential batch separation with DEAE-cellulose, hydrophobic interaction chromatography with octyl-Sepharose, and ion-exchange chromatography with DEAE-Bio-Gel A (Bio-Rad Laboratories, Richmond, Calif.). The resulting protease preparation was determined to be homogeneous by polyacrylamide gel electrophoresis in the presence and absence of sodium dodecyl sulfate. Although free of contaminating proteins, the purified protease separated into two antigenically indistinguishable proteins both of which possessed proteolytic activity. The apparent masses of the proteins were 38 and 40 kilodaltons (kDa) as determined by polyacrylamide gel electrophoresis in sodium dodecyl sulfate, whereas gel filtration chromatography revealed a single mass of 34 kDa. Immunoblot analysis indicated that the 38-kDa protein probably originated from the 40-kDa protein during purification. The isoelectric points of the two protease species were 4.20 and 4.42. Enzyme activity, which was optimum between pH 5.5 and 7.5, was inhibited by various metal chelators; however, no effect was observed after treatment with phenylmethylsulfonyl fluoride, chymostatin, trypsin inhibitor, or dithiothreitol. Enzyme activity inhibited by metal chelators was restored upon the addition of various metal ions, including Zn2+, Fe2+, Mn2+, Cu2+, and Fe3+, but was not restored by Mg2+ or Ca2+. Atomic absorption analysis of the purified protease revealed a single gram-atom of zinc per mole of enzyme. Our findings indicate that the L. pneumophila protease resembles neutral zinc-containing metalloproteases similar to those found in other bacterial species.
Full text
PDF


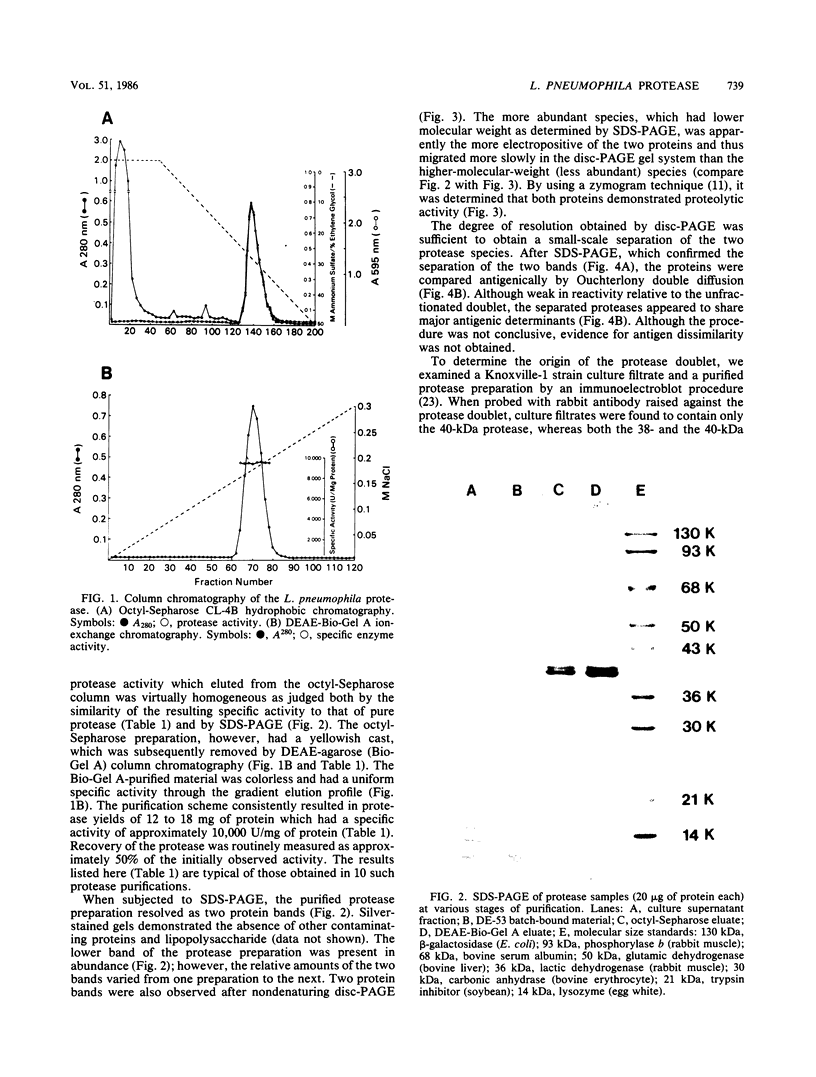

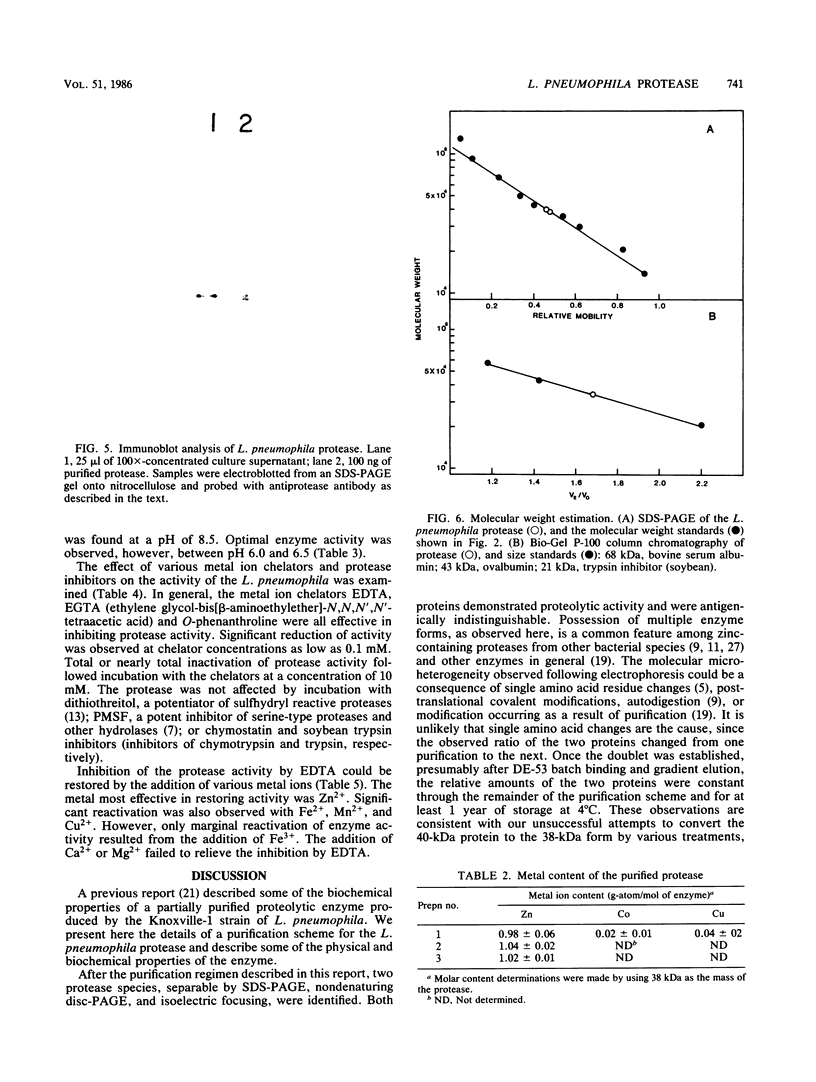

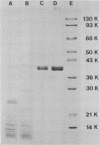
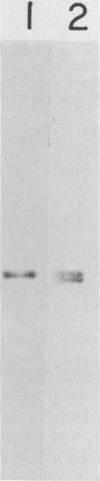
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berdal B. P., Fossum K. Occurrence and immunogenicity of proteinases from Legionella species. Eur J Clin Microbiol. 1982 Feb;1(1):7–11. doi: 10.1007/BF02014133. [DOI] [PubMed] [Google Scholar]
- Berdal B. P., Hushovd O., Olsvik O., odegård O. R., Bergan T. Demonstration of extracellular proteolytic enzymes from Legionella species strains by using synthetic chromogenic peptide substrates. Acta Pathol Microbiol Immunol Scand B. 1982 Apr;90(2):119–123. doi: 10.1111/j.1699-0463.1982.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Berdal B. P., Olsvik O., Myhre S., Omland T. Demonstration of extracellular chymotrypsin-like activity from various Legionella species. J Clin Microbiol. 1982 Sep;16(3):452–457. doi: 10.1128/jcm.16.3.452-457.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Edelstein P. H. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J Clin Microbiol. 1981 Sep;14(3):298–303. doi: 10.1128/jcm.14.3.298-303.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger A. S., Gray L. D. Purification of Pseudomonas aeruginosa proteases and microscopic characterization of pseudomonal protease-induced rabbit corneal damage. Infect Immun. 1978 Feb;19(2):630–648. doi: 10.1128/iai.19.2.630-648.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lyerly D., Kreger A. Purification and characterization of a Serratia marcescens metalloprotease. Infect Immun. 1979 May;24(2):411–421. doi: 10.1128/iai.24.2.411-421.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayaux J. F., Kalogerakos T., Brito K. K., Blanquet S. Removal of the tightly bound zinc from Escherichia coli trypsin-modified methionyl-tRNA synthetase. Eur J Biochem. 1982 Nov;128(1):41–46. doi: 10.1111/j.1432-1033.1982.tb06928.x. [DOI] [PubMed] [Google Scholar]
- Morihara K. The specificities of various neutral and alkaline proteinases from microorganisms. Biochem Biophys Res Commun. 1967 Mar 21;26(6):656–661. doi: 10.1016/s0006-291x(67)80122-0. [DOI] [PubMed] [Google Scholar]
- Müller H. E. Enzymatic profile of Legionella pneumophilia. J Clin Microbiol. 1981 Mar;13(3):423–426. doi: 10.1128/jcm.13.3.423-426.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H. E. Proteolytic action of Legionella pneumophila on human serum proteins. Infect Immun. 1980 Jan;27(1):51–53. doi: 10.1128/iai.27.1.51-53.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht H., Geokas M. C., Silverman P., Haverback B. J. A new ultrasensitive method for the determination of proteolytic activity. Clin Chim Acta. 1968 Aug;21(2):197–203. doi: 10.1016/0009-8981(68)90127-7. [DOI] [PubMed] [Google Scholar]
- Ristroph J. D., Hedlund K. W., Allen R. G. Liquid medium for growth of Legionella pneumophila. J Clin Microbiol. 1980 Jan;11(1):19–21. doi: 10.1128/jcm.11.1.19-21.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susor W. A., Kochman M., Rutter W. J. Heterogeneity of presumably homogeneous protein preparations. Science. 1969 Sep 19;165(3899):1260–1262. doi: 10.1126/science.165.3899.1260. [DOI] [PubMed] [Google Scholar]
- Swann J. C., Reynolds J. J., Galloway W. A. Zinc metalloenzyme properties of active and latent collagenase from rabbit bone. Biochem J. 1981 Apr 1;195(1):41–49. doi: 10.1042/bj1950041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M. R., Miller R. D., Iglewski B. H. In vitro production of an extracellular protease by Legionella pneumophila. Infect Immun. 1981 Oct;34(1):299–302. doi: 10.1128/iai.34.1.299-302.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe T. C., Miller R. D. Extracellular enzymes of Legionella pneumophila. Infect Immun. 1981 Aug;33(2):632–635. doi: 10.1128/iai.33.2.632-635.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS D. E., REISFELD R. A. DISC ELECTROPHORESIS IN POLYACRYLAMIDE GELS: EXTENSION TO NEW CONDITIONS OF PH AND BUFFER. Ann N Y Acad Sci. 1964 Dec 28;121:373–381. doi: 10.1111/j.1749-6632.1964.tb14210.x. [DOI] [PubMed] [Google Scholar]
- Warren W. J., Miller R. D. Growth of Legionnaires disease bacterium (Legionella pneumophila) in chemically defined medium. J Clin Microbiol. 1979 Jul;10(1):50–55. doi: 10.1128/jcm.10.1.50-55.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Broadbent D. A. Biochemical characterization of extracellular proteases from Vibrio cholerae. Infect Immun. 1982 Sep;37(3):875–883. doi: 10.1128/iai.37.3.875-883.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong W. W., Zweers A., Cohen L. H. Influence of single amino acid substitutions on electrophoretic mobility of sodium dodecyl sulfate-protein complexes. Biochem Biophys Res Commun. 1978 May 30;82(2):532–539. doi: 10.1016/0006-291x(78)90907-5. [DOI] [PubMed] [Google Scholar]